| Citation: Xu Nan, Wu Cai-lai, Li Sheng-rong, Xue Bo-qiang, He Xiang, Yu Yan-long, Liu Jun-zhuang. 2020. LA-ICP-MS in situ analyses of the pyrites in Dongyang gold deposit, Southeast China: Implications to the gold mineralization. China Geology, 3(2), 230‒246. doi: 10.31035/cg2018123. |
Pyrite is an important sulfide mineral in most hydrothermal deposits (Keith M et al., 2016a; Rickard D and Luther GW, 2007), which is stable in various physicochemical conditions (Deditius A et al., 2014; Cook NJ et al., 2009a). The refractory (Wohlgemuth-Ueberwasser CC et al., 2015) and common occurrence make pyrite suitable to reconstruct ore-forming processes (Large RR et al., 2009; Agangi A et al., 2013). Pyrite chemistry has a systematic relationship to the metal sources (Wohlgemuth-Ueberwasser CC et al., 2015), which is an indicator of fluid composition (Deditius AP et al., 2014; Keith M et al., 2016a; Reich M et al., 2013), and can be used to reveal the ore-forming processes in many hydrothermal systems (Reich M et al., 2013), such as porphyry-epithermal and volcanic-hosted deposits (Deditius AP et al., 2014; Keith M et al., 2016b). The enrichment of trace elements is variable in deposits (Seedorff E et al., 2005; Goldfarb RJ et al., 2005), and can be significantly fractionated in different hydrothermal processes (Cook NJ et al., 2009b; Reich M et al., 2005). The variation of trace elements in pyrite was usually caused by temperature, pH and redox, fluid-wall rock interaction and phase separation (Revan MK et al., 2014; Keith M et al., 2016a, 2016b; Chouinard A et al., 2005).
Tellurium and Au hosted in pyrite have been identified as economic interest elements in epithermal deposits (Simmons SF et al., 2005; Kesler SE et al., 2007; Deditius AP et al., 2014), resulting from their ubiquity and ability to concentrate trace elements (Reich M et al., 2013; Cline JS et al., 2005). Significant amount of trace elements is usually incorporated into nanoparticles in hydrothermal pyrite, which is most common in hydrothermal systems, especially low temperature deposits (Deditius AP et al., 2014). Previous research highlight the importance of many elements sinked in pyrite (Keith M et al., 2016b; Maslennikov VV et al., 2009), such as Au, Ag, Pb, As, Co, Ni, etc. (Reich M et al., 2005; Smith JW et al., 2014; Deditius A et al., 2009a), and most of these studies focused on As (King J et al., 2014; Large RR et al., 2009; Keith M et al., 2016a), while rarely involved Te (Keith M et al., 2016b), which might include details to the ore-forming processes (Reich M et al., 2013; Deditius AP et al., 2014).
Epithermal mineralization is generally controlled by cooling, condensation of magmatic vapours, fluid boiling and incorporation of magmatic and meteoric fluids (Seedorff E et al., 2005; Simmons SF et al., 2005; Kouzmanov K et al., 2010), while metal elements are often fractionated in porphyry and epithermal deposits (Deditius AP et al., 2014). Gold generally stays in solution in deeper porphyry environment, and precipitates at shallower crust in epithermal conditions (< 1.5 km, < 300°C; Simmons SF et al., 2005), which explains the lower Au grades in porphyry compared to epithermal systems (Deditius AP et al., 2014; Keith M et al., 2017). Magmatic origin fluids are in high temperature (up to 300°C) and high-S epithermal systems (Einaudi MT et al., 2003), and are oxidized and more acidic (Sillitoe RH and Hedenquist JW, 2003), while shallower low temperature (< 200°C) fluids in low-S epithermal systems are reduced and near neutral in pH, due to interaction between host rocks and fluid, as well as high meteoric fluid mixing (Simmons SF et al., 2005). These differences lead to systematic variation in the composition of high- and low-S epithermal deposits, such as higher Cu contents and higher abundances of sulfosalts compared to low-S deposits (Einaudi MT et al., 2003).
The Dongyang gold deposit is a newly discovered ore deposit in Southeast China, belonging to the Circum-Pacific polymetallic metallogenic belt. The authors study focused on the genesis and origin of the deposit (Xu N et al., 2017, 2020), but the regularity of gold concentration and precipitation are still unclear. Herewith, the paper presents mineralogical, SEM and LA-ICP-MS analysis to reveal the relations between Au and Te, As, S and Fe, etc., within the arsenian pyrite, and discuss the gold precipitation process.
Dongyang gold deposit was located at Fujian Province, Southeast China (Fig. 1a–d), close to the southeastern margin of the Asian continent, and also interaction zone of the Eurasian and Pacific Plate. The basement in this region is mainly composed of Paleo-Mesoproterozoic metamorphic rocks, such as Gekeng Formation and the Daoxiang Formation (Fig. 1c), the former was dominated by Mesoproterozoic mica schist and quartz schist, while the later was by Late Proterozoic metavolcanics and metasandstone. Those units were almost covered by Mesozoic volcaniclastic rocks of the Nanyuan and Changlin Formations, composed of rhyolitic tuff and dacite tuff (Fig. 1c). Multiple tectonic activities occurred from Proterozoic to Cenozoic, and the Yanshanian (Mesozoic) tectonic-magmatic events was the most significant, which was caused by the subduction of Pacific Plate beneath the Eurasian Plate, and characterized by large scale intermediate-acid intrusive rocks and dykes, such as diorite, quartz monzonite, granodiorite and monzonitic granite (Fig. 1c), etc.
The research region was dominated by the Nanyuan Formation and Changlin Formation (Fig. 1d). The former is mainly composed of rhyolitic tuff, dacite tuff, dacite-rhyolitic tuff, tuffaceous breccias and volcanic breccias, while the latter is composed of conglomerates, breccias, gritstone and greywacke, as well as tuffaceous siltstone. Many faults in direction of NE and NW exist in this region (Fig. 1d), and the later are main ore-control structures. The sub-rhyolite porphyry, which was main ore-bearing dykes, was hosted along the NW fault (Fig. 1d), and the ore bodies were mostly developed in the dykes, or in the contact zones to the country rocks, with Au grade in the range of 3.3–6.96 g/t and 2.9–3.9 g/t, respectively. The ores are mainly of primary ore, including sub-rhyolite porphyry and breccias. The former are of banded structure with many small ore-bearing veins filling in its fractures, and the later exist in the contact zones with some ore-bearing veins. According to field geological investigations and petrographic studies, the authors divide the mineralization into four stages: (1) Sericitization stage (stage i); (2) gold-silicification-pyritization stage (stage ii); (3) silicification-polymetallic sulfide stage (stage iii); (4) Quartz-carbonation stage (stage vi). Among these, stages ii and iii are the main metallogenic stages, and the mineral associations are sericite+illite+kaolinite, gold+pyrite, pyrite+sphalerite+chalcopyrite+gold/silver bearing minerals+other sulfide minerals, and quartz+carbonatite, respectively. Alteration types are mainly silicification and sericitization, with subordinate argillization and carbonation. The alteration zoning is identified as silicification + sericitization → argillization → chloritization+epidotization from the centre of ore body to its periphery (Xu N et al., 2017). The gangue minerals include sericite and quartz, as well as some calcite. Feldspar show sericitization, preserving the original crystal shape. The metallic minerals are mainly of pyrite, and little sphalerite, chalcopyrite and galena. According to backscatter images, many other metal inclusions were also observed in the fractures of pyrite, including allargentum (Figs. 2e, f, j, i), chalcopyrite (Figs. 2b, d, f, g, h, i), electrum (Figs. 2a, c), tetrahedrite (Figs. 2a–i), galena (Figs. 2d, j, k), hessite (Fig. 2j), polybasite (Fig. 2c), sphalerite (Fig. 2k), stephanite (Figs. 2h, i) and silver (Fig. 2e). The exsolution structure indicates sphalerite crystallized earlier than chalcopyrite (Figs. 3d–f), while the intergrowth of sphalerite and galena imply they were formed in simultaneously (Fig. 2k). Tetrahedrite, allargentum and silver (Figs. 2e–g) always show paragenetic relationship, as well as stephanite, allargentum and tetrahedrite (Figs. 2h, i), suggesting these minerals crystallized at the same stage, and the replacement of tetrahedrite to chalcopyrite (Figs. 2g–i) imply chalcopyrite crystalized earlier than those minerals. The precipitation of electrum was earlier than tetrahedrite, as the later always existed along the fracture of the former (Figs. 2a, c). The crystal form of pyrite includes cube crystal (Figs. 4a–c), pyritohedron (Fig. 4j), and octahedral crystal (Fig. 4k). Subhedral and xenomorphic pyrite are also seen under the microscope (Figs. 4d–i). In this study, the authors divide the pyrite into three groups (Py I, Py II and Py III), representing euhedral, subhedral and anhedral pyrite, respectively, based on pyrite geochemistry and mineralogy. The Py III always formed along with the Py I/Py II (Figs. 4f, i), indicating that these pyrites crystallized later than the Py I and the Py II. Sphalerite, chalcopyrite and galena were always observed in the fractures of the Py I (Figs. 3a, i), indicating these minerals crystallized later than the Py I, and the intergrowth of sphalerites and the Py II were always seen under microscope (Fig.3d), indicating the Py II were generated later the Py III. Thus, the sequence of pyrite formation for the three types pyrite was Py I → Py II → Py III. All in all, the authors propose that the metal minerals crystallized in order of pyrite, to sphalerite + galena, to chalcopyrite + electrum, and to tetrahedrite + allargentum + silver + stephanite + polybasite.
Seventeen samples were selected from the ores in drill cores along the exploring line 207, in the Jitou ore section, including drills ZK2071, ZK2072, ZK2073 and ZK2076, as listed in Table 1. A total of 40 polished thin sections were prepared for mineralogy, SEM and LA-ICP-MS analysis.
| NO. | Stage | S/% | Fe/% | Cu | Zn | Pb | Co | Ni | As | Se | Mg | Al | Mn |
| 117-2 | Py I | 52.78 | 45.68 | 52.81 | 3.08 | 214.96 | 630.02 | 150.03 | 5160.69 | 5.83 | 28.70 | 309.21 | 20.16 |
| 240-1 | Py I | 54.29 | 39.05 | 253.62 | 119.85 | 499.34 | 80.67 | 17.82 | 2828.20 | 0.00 | 6918.19 | 20681.55 | 308.34 |
| 240-3 | Py I | 49.09 | 42.32 | 46.54 | 47.67 | 726.41 | 265.47 | 23.17 | 1681.40 | 0.00 | 4871.61 | 8165.10 | 5740.33 |
| 340-1 | Py I | 51.15 | 47.48 | 169.77 | 3.15 | 66.12 | 276.34 | 85.30 | 2316.20 | 9.71 | 25.66 | 491.92 | 8.73 |
| 3128-2 | Py I | 52.55 | 47.09 | 3.85 | 0.52 | 23.82 | 14.98 | 58.15 | 1556.58 | 11.54 | 0.47 | 1.21 | 0.15 |
| 13-2 | Py II | 53.36 | 44.97 | 6.56 | 4.94 | 21.56 | 2.93 | 2.05 | 13040.62 | 2.95 | 27.71 | 404.55 | 2.66 |
| 13-21 | Py II | 51.77 | 47.85 | 5.05 | 1.24 | 16.97 | 12.55 | 5.19 | 3220.99 | 4.30 | 6.23 | 44.08 | 1.88 |
| 13-24 | Py II | 50.89 | 48.33 | 11.20 | 2.57 | 23.25 | 12.25 | 5.44 | 1605.86 | 0.00 | 23.96 | 213.27 | 9.49 |
| 117-3 | Py II | 53.72 | 45.68 | 8.89 | 2.60 | 22.82 | 86.51 | 24.56 | 1990.04 | 2.55 | 3.70 | 333.95 | 1.40 |
| 125-1 | Py II | 54.13 | 41.54 | 418.89 | 36675.19 | 319.32 | 31.68 | 6.43 | 3175.61 | 0.00 | 141.83 | 514.67 | 241.28 |
| 125-2 | Py II | 55.07 | 44.42 | 18.32 | 0.00 | 69.54 | 217.15 | 2.20 | 2720.92 | 0.49 | 12.10 | 430.78 | 11.79 |
| 125-22 | Py II | 54.89 | 44.79 | 24.01 | 2.70 | 65.31 | 4.41 | 4.98 | 960.04 | 0.56 | 21.29 | 486.88 | 48.83 |
| 179-1 | Py II | 53.87 | 45.44 | 35.58 | 1.13 | 171.04 | 0.21 | 0.38 | 862.34 | 0.00 | 65.84 | 1097.15 | 18.31 |
| 22-1 | Py II | 52.17 | 47.37 | 15.65 | 0.73 | 27.22 | 1108.32 | 3113.78 | 8.54 | 0.59 | 0.12 | 0.31 | 0.38 |
| 22-12 | Py II | 52.81 | 46.57 | 80.37 | 0.79 | 24.90 | 1446.51 | 4240.78 | 9.74 | 0.00 | 1.43 | 9.24 | 0.23 |
| 310-3 | Py II | 50.36 | 49.56 | 8.01 | 0.66 | 29.22 | 4.24 | 1.07 | 330.73 | 0.00 | 4.18 | 3.81 | 1.72 |
| 360-1 | Py II | 52.99 | 45.20 | 37.05 | 4.59 | 7.46 | 3.40 | 7.45 | 17227.57 | 8.38 | 11.85 | 165.11 | 1.15 |
| 360-2 | Py II | 55.32 | 42.28 | 59.01 | 17.30 | 177.71 | 781.21 | 201.18 | 1966.23 | 11.51 | 410.73 | 3692.21 | 131.23 |
| 3127-3 | Py II | 51.54 | 46.92 | 22.86 | 10.66 | 425.29 | 2642.24 | 2156.33 | 4720.39 | 4.36 | 879.64 | 20.11 | 347.58 |
| 3128-11 | Py II | 55.31 | 41.83 | 30.47 | 2.16 | 61.53 | 18.27 | 58.95 | 28097.12 | 3.13 | 5.76 | 18.59 | 0.39 |
| 3128-12 | Py II | 57.23 | 42.29 | 31.33 | 5.99 | 48.02 | 0.57 | 8.40 | 2550.44 | 3.91 | 48.53 | 471.02 | 2.75 |
| 614-11 | Py II | 56.05 | 43.10 | 89.30 | 1.60 | 99.31 | 1163.01 | 259.55 | 2868.70 | 0.00 | 10.82 | 507.95 | 4.30 |
| 614-12 | Py II | 55.62 | 43.86 | 360.68 | 1331.71 | 262.02 | 50.10 | 38.84 | 406.05 | 11.52 | 11.87 | 41.50 | 10.47 |
| 13-11 | Py III | 53.36 | 46.15 | 20.71 | 4.62 | 124.93 | 163.38 | 9.23 | 1267.27 | 0.00 | 76.52 | 369.37 | 36.95 |
| 13-12 | Py III | 53.11 | 46.35 | 15.51 | 0.99 | 88.77 | 42.47 | 3.08 | 394.47 | 0.69 | 10.19 | 682.65 | 32.07 |
| 13-22 | Py III | 51.77 | 47.85 | 5.05 | 1.24 | 16.97 | 12.55 | 5.19 | 3220.99 | 4.30 | 6.23 | 44.08 | 1.88 |
| 13-23 | Py III | 52.67 | 47.02 | 5.88 | 0.00 | 17.78 | 15.30 | 6.60 | 1495.93 | 1.39 | 20.76 | 89.73 | 9.02 |
| 181-1 | Py III | 56.93 | 42.66 | 12.15 | 3.11 | 79.69 | 268.00 | 3.65 | 1598.41 | 0.00 | 94.84 | 274.96 | 44.53 |
| 27-11 | Py III | 53.72 | 43.56 | 7.93 | 0.00 | 14.70 | 153.84 | 113.03 | 664.25 | 0.38 | 4988.44 | 27.43 | 2240.83 |
| 27-12 | Py III | 54.09 | 45.17 | 18.58 | 8.94 | 38.40 | 157.84 | 93.67 | 336.19 | 0.58 | 683.06 | 757.85 | 337.57 |
| 27-13 | Py III | 53.09 | 43.29 | 27.25 | 26.89 | 65.26 | 85.06 | 77.18 | 107.31 | 0.00 | 4249.11 | 3404.40 | 2761.94 |
| 214-1 | Py III | 56.08 | 43.79 | 2.44 | 0.83 | 7.71 | 21.56 | 34.17 | 52.09 | 0.26 | 20.99 | 252.63 | 1.39 |
| 214-21 | Py III | 54.83 | 44.88 | 16.40 | 1.33 | 53.65 | 81.01 | 87.85 | 132.52 | 2.05 | 8.10 | 455.44 | 0.74 |
| 214-22 | Py III | 55.61 | 43.80 | 21.63 | 0.00 | 67.73 | 156.11 | 208.21 | 190.55 | 0.00 | 10.65 | 1012.42 | 1.63 |
| 214-23 | Py III | 53.00 | 46.86 | 12.42 | 0.86 | 39.56 | 140.67 | 166.13 | 118.08 | 3.63 | 14.35 | 216.99 | 1.72 |
| 230-1 | Py III | 55.72 | 43.81 | 30.44 | 0.34 | 131.96 | 88.97 | 11.85 | 3536.04 | 1.54 | 11.76 | 65.40 | 16.50 |
| 230-3 | Py III | 55.48 | 44.21 | 14.58 | 1.44 | 47.73 | 5.46 | 5.01 | 2092.6 | 2.64 | 20.82 | 54.50 | 2.23 |
| 34-11 | Py III | 51.22 | 48.63 | 5.37 | 3.32 | 72.82 | 139.02 | 14.48 | 293.53 | 1.87 | 11.40 | 149.28 | 11.09 |
| 360-2 | Py III | 55.32 | 42.28 | 59.01 | 17.3 | 177.71 | 781.21 | 201.18 | 1966.23 | 11.51 | 410.73 | 3692.21 | 131.23 |
| 382-22 | Py III | 54.14 | 44.75 | 104.49 | 7.54 | 56.94 | 881.75 | 217.24 | 1167.76 | 1.62 | 139.88 | 2351.25 | 18.74 |
| 3127-2 | Py III | 53.59 | 46.33 | 0.27 | 0.64 | 0.67 | 100.37 | 241.13 | 19.20 | 5.75 | 1.09 | 16.47 | 1.21 |
| 3128-21 | Py III | 50.56 | 48.03 | 28.40 | 0.24 | 18.09 | 2104.00 | 650.62 | 10398.53 | 14.03 | 0.00 | 0.00 | 0.10 |
| 3128-22 | Py III | 50.74 | 46.85 | 165.86 | 1.72 | 2589.79 | 1351.22 | 1844.08 | 15522.4 | 7.90 | 9.10 | 62.13 | 1.54 |
| 614-3 | Py III | 52.19 | 47.51 | 6.98 | 0.00 | 37.15 | 0.75 | 0.16 | 130.46 | 0.35 | 35.29 | 247.98 | 15.22 |
The mineralogy observation and SEM analysis were carried out at the Mineralogy Laboratory of China University of Geosciences (Beijing) using Leica 4500P petrological microscope and Mira XMU, respectively. Spectrum work distance is 15 mm, with voltage of 20 kV, current of 10 nA and signal intensity of 5000 Cps.
The pyrite geochemistry was carried out in LA-ICP-MS Lab in Nanjing University. In situ analyses of single pyrite crystals were done by LA-ICP-MS transects across pyrite crystals in polished thin sections, using Agilent 7500a ICP-MS on the GeoLas 2005. Elements used for the analysis are Au, S, Fe, Ag, Cu, Zn, Pb, Co, Ni, As, Se, Mg, Al, Mn, Ti, Cr, Zr, Mo, Sb, Te, Hf, W, Bi, Th, U and REEs, as listed in Table 1. Analyses were performed in He atmosphere, using a pulse rate of 8 Hz and beam energy of 6 J/cm2 at the samples. Laser spot size was 20–40 μm in diameter. Analyses were begun in 30 seconds during which no material was ablated to establish a background reading. Detection limits were determined as 3 times the average of this background reading. Laflamme Po726 was used as a reference material for standardisation prior to, during, and after analyses and was used to correct for instrument drift throughout analyses. The result data was analyzed by the software of ICP-MS DataCal.
Details of the pyrite geochemistry analyses of the Dongyang gold deposit was presented in Table 1. Inter-element correlation coefficients are presented in Table 2, Table 3 and Table 4.
| Au | S | Fe | Ag | Cu | Zn | Pb | Co | Ni | As | Se | Mg | Al | Mn | Ti | Cr | Mo | Sb | Te | W | Bi | |
| Au | 1.00 | ||||||||||||||||||||
| S | 0.23 | 1.00 | |||||||||||||||||||
| Fe | 0.29 | −0.23 | 1.00 | ||||||||||||||||||
| Ag | 0.99# | 0.25 | 0.37 | 1.00 | |||||||||||||||||
| Cu | −0.45 | 0.44 | −0.55 | −0.50 | 1.00 | ||||||||||||||||
| Zn | −0.22 | 0.35 | −0.96# | −0.30 | 0.70 | 1.00 | |||||||||||||||
| Pb | −0.44 | −0.34 | −0.82 | −0.51 | 0.18 | 0.68 | 1.00 | ||||||||||||||
| Co | −0.66 | −0.15 | 0.24 | −0.58 | −0.21 | −0.39 | 0.00 | 1.00 | |||||||||||||
| Ni | −0.22 | 0.14 | 0.64 | −0.11 | −0.31 | −0.69 | −0.58 | 0.81 | 1.00 | ||||||||||||
| As | −0.49 | 0.42 | 0.02 | −0.41 | 0.07 | −0.10 | −0.12 | 0.83 | 0.78 | 1.00 | |||||||||||
| Se | 0.54 | 0.08 | 0.91* | 0.60 | −0.38 | −0.81 | −0.95* | −0.07 | 0.49 | −0.09 | 1.00 | ||||||||||
| Mg | −0.26 | 0.08 | −0.97# | −0.35 | 0.54 | 0.95* | 0.85 | −0.36 | −0.77 | −0.21 | −0.90* | 1.00 | |||||||||
| Al | −0.22 | 0.35 | −0.96# | −0.30 | 0.71 | 1.00# | 0.68 | −0.39 | −0.69 | −0.10 | −0.80 | 0.95* | 1.00 | ||||||||
| Mn | −0.25 | −0.80 | −0.36 | −0.31 | −0.28 | 0.19 | 0.81 | 0.01 | −0.48 | −0.39 | −0.60 | 0.47 | 0.19 | 1.00 | |||||||
| Ti | −0.36 | 0.26 | 0.16 | −0.27 | −0.24 | −0.29 | −0.14 | 0.88 | 0.84 | 0.95* | 0.00 | −0.35 | −0.30 | −0.27 | 1.00 | ||||||
| Cr | −0.40 | −0.69 | −0.55 | −0.46 | −0.03 | 0.40 | 0.91* | −0.02 | −0.57 | −0.35 | −0.76 | 0.65 | 0.40 | 0.96# | −0.30 | 1.00 | |||||
| Mo | −0.30 | 0.37 | −0.87 | −0.38 | 0.87 | 0.95* | 0.54 | −0.40 | −0.65 | −0.12 | −0.69 | 0.87 | 0.95* | 0.05 | −0.37 | 0.29 | 1.00 | ||||
| Sb | −0.30 | 0.46 | −0.79 | −0.37 | 0.92* | 0.91* | 0.41 | −0.39 | −0.57 | −0.07 | −0.59 | 0.79 | 0.91* | −0.09 | −0.34 | 0.16 | 0.98# | 1.00 | |||
| Te | 0.77 | 0.34 | 0.51 | 0.83 | −0.67 | −0.52 | −0.60 | −0.04 | 0.40 | 0.13 | 0.62 | −0.59 | −0.53 | −0.44 | 0.31 | −0.62 | −0.64 | −0.61 | 1.00 | ||
| W | −0.36 | 0.22 | 0.21 | −0.27 | −0.27 | −0.35 | −0.17 | 0.89* | 0.87 | 0.94* | 0.04 | −0.40 | −0.35 | −0.26 | 0.99# | −0.30 | −0.42 | −0.38 | 0.31 | 1.00 | |
| Bi | −0.34 | 0.28 | 0.16 | −0.25 | −0.24 | −0.29 | −0.15 | 0.86 | 0.84 | 0.95* | 0.00 | −0.35 | −0.29 | −0.28 | 1.00# | −0.31 | −0.37 | −0.34 | 0.33 | 0.99# | 1.00 |
| Note: #, correlation is significant at the 0.01 level (2-tailed). *, correlation is significant at the 0.05 level (2-tailed). | |||||||||||||||||||||
| Au | S | Fe | Ag | Cu | Zn | Pb | Co | Ni | As | Se | Mg | Al | Mn | Ti | Cr | Mo | Sb | Te | W | Bi | |
| Au | 1.00 | ||||||||||||||||||||
| S | 0.28 | 1.00 | |||||||||||||||||||
| Fe | −0.40 | −0.88# | 1.00 | ||||||||||||||||||
| Ag | 0.02 | −0.24 | 0.14 | 1.00 | |||||||||||||||||
| Cu | −0.09 | 0.29 | −0.46 | −0.06 | 1.00 | ||||||||||||||||
| Zn | −0.08 | 0.06 | −0.38 | −0.05 | 0.75# | 1.00 | |||||||||||||||
| Pb | −0.03 | 0.10 | −0.29 | 0.70# | 0.58* | 0.46 | 1.00 | ||||||||||||||
| Co | −0.04 | −0.19 | 0.16 | 0.77# | −0.10 | −0.14 | 0.46 | 1.00 | |||||||||||||
| Ni | −0.10 | −0.30 | 0.31 | 0.30 | −0.09 | −0.11 | 0.05 | 0.73# | 1.00 | ||||||||||||
| As | 0.78# | 0.13 | −0.34 | −0.02 | −0.15 | −0.06 | −0.16 | −0.19 | −0.22 | 1.00 | |||||||||||
| Se | 0.01 | 0.27 | −0.30 | 0.15 | 0.23 | −0.18 | 0.25 | −0.01 | −0.20 | 0.19 | 1.00 | ||||||||||
| Mg | −0.02 | −0.15 | −0.02 | 0.92# | −0.01 | 0.05 | 0.75# | 0.71# | 0.22 | −0.06 | 0.28 | 1.00 | |||||||||
| Al | −0.13 | 0.34 | −0.40 | −0.05 | −0.01 | 0.01 | 0.18 | 0.01 | −0.21 | −0.15 | 0.42 | 0.30 | 1.00 | ||||||||
| Mn | −0.06 | −0.12 | −0.16 | 0.80# | 0.33 | 0.49* | 0.86# | 0.56* | 0.15 | −0.09 | 0.11 | 0.88# | 0.20 | 1.00 | |||||||
| Ti | −0.11 | 0.25 | −0.14 | −0.07 | 0.60# | 0.01 | 0.31 | −0.20 | −0.18 | −0.15 | 0.55* | −0.12 | −0.09 | −0.10 | 1.00 | ||||||
| Cr | −0.01 | 0.25 | −0.30 | 0.54* | 0.13 | −0.10 | 0.57* | 0.37 | 0.00 | −0.06 | 0.75# | 0.73# | 0.60# | 0.54* | 0.25 | 1.00 | |||||
| Mo | −0.12 | 0.06 | −0.39 | −0.07 | 0.70# | 0.97# | 0.43 | −0.17 | −0.17 | −0.07 | −0.14 | 0.08 | 0.14 | 0.50* | −0.02 | −0.05 | 1.00 | ||||
| Sb | −0.17 | 0.33 | −0.37 | 0.22 | 0.63# | 0.43 | 0.74# | 0.12 | −0.14 | −0.30 | 0.04 | 0.26 | 0.17 | 0.41 | 0.39 | 0.21 | 0.38 | 1.00 | |||
| Te | 0.01 | −0.27 | 0.17 | 0.99# | −0.07 | 0.65# | 0.75# | 0.30 | −0.02 | 0.13 | 0.91# | −0.05 | 0.77# | −0.09 | 0.52* | −0.10 | 0.15 | 1.00 | |||
| W | −0.13 | 0.27 | −0.24 | −0.07 | 0.78# | 0.26 | 0.42 | −0.20 | −0.18 | −0.18 | 0.48* | −0.10 | −0.10 | 0.04 | 0.96# | 0.22 | 0.23 | 0.46 | −0.10 | 1.00 | |
| Bi | −0.01 | −0.06 | 0.05 | 0.75# | −0.08 | −0.14 | 0.52* | 0.56* | 0.13 | −0.11 | 0.22 | 0.70# | 0.08 | 0.54* | 0.11 | 0.49* | −0.16 | 0.16 | 0.81# | 0.06 | 1.00 |
| Note: #, correlation is significant at the 0.01 level (2-tailed). *, correlation is significant at the 0.05 level (2-tailed). | |||||||||||||||||||||
| Au | S | Fe | Ag | Cu | Zn | Pb | Co | Ni | As | Se | Mg | Al | Mn | Ti | Cr | Mo | Sb | Te | W | Bi | |
| Au | 1.00 | ||||||||||||||||||||
| S | −0.37 | 1.00 | |||||||||||||||||||
| Fe | 0.17 | −0.85# | 1.00 | ||||||||||||||||||
| Ag | 1.0# | −0.38 | 0.17 | 1.00 | |||||||||||||||||
| Cu | 0.81# | −0.22 | −0.04 | 0.81# | 1.00 | ||||||||||||||||
| Zn | −0.07 | 0.07 | −0.43 | −0.07 | 0.19 | 1.00 | |||||||||||||||
| Pb | 0.99# | −0.35 | 0.14 | 0.99# | 0.82# | −0.04 | 1.00 | ||||||||||||||
| Co | 0.45* | −0.41 | 0.18 | 0.45* | 0.61# | 0.04 | 0.44* | 1.00 | |||||||||||||
| Ni | 0.93# | −0.45* | 0.21 | 0.93# | 0.82# | −0.06 | 0.92# | 0.70# | 1.00 | ||||||||||||
| As | 0.81# | −0.48* | 0.28 | 0.80# | 0.70# | −0.15 | 0.79# | 0.79# | 0.89# | 1.00 | |||||||||||
| Se | 0.31 | −0.39 | 0.22 | 0.31 | 0.36 | 0.05 | 0.30 | 0.79# | 0.54* | 0.64# | 1.00 | ||||||||||
| Mg | −0.09 | −0.02 | −0.38 | −0.09 | −0.08 | 0.47* | −0.09 | −0.12 | −0.09 | −0.17 | −0.22 | 1.00 | |||||||||
| Al | −0.13 | 0.20 | −0.51* | −0.12 | 0.29 | 0.88# | −0.09 | 0.11 | −0.08 | −0.20 | 0.11 | 0.32 | 1.00 | ||||||||
| Mn | −0.09 | −0.04 | −0.37 | −0.08 | −0.08 | 0.57# | −0.09 | −0.13 | −0.09 | −0.17 | −0.23 | 0.98# | 0.39 | 1.00 | |||||||
| Ti | −0.21 | 0.19 | −0.07 | −0.20 | −0.22 | −0.04 | −0.19 | −0.30 | −0.25 | −0.28 | −0.13 | −0.05 | −0.04 | −0.04 | 1.00 | ||||||
| Cr | 0.02 | 0.22 | −0.39 | 0.01 | 0.37 | 0.46* | 0.06 | 0.29 | 0.06 | 0.04 | 0.44* | −0.08 | 0.70# | −0.11 | −0.06 | 1.00 | |||||
| Mo | 0.99# | −0.38 | 0.16 | 0.99# | 0.81# | −0.05 | 0.99# | 0.47* | 0.94# | 0.81# | 0.34 | −0.08 | −0.10 | −0.08 | −0.20 | 0.04 | 1.00 | ||||
| Sb | 0.80# | −0.06 | −0.07 | 0.80# | 0.69# | −0.08 | 0.82# | 0.27 | 0.75# | 0.60# | 0.15 | −0.12 | −0.05 | −0.11 | 0.07 | 0.01 | 0.80# | 1.00 | |||
| Te | 0.99# | −0.42 | 0.20 | 0.99# | 0.81# | −0.07 | 0.98# | 0.55# | 0.96# | 0.87# | 0.41 | −0.09 | −0.12 | −0.09 | −0.23 | 0.03 | 0.99# | 0.78# | 1.00 | ||
| W | 0.42 | 0.10 | −0.02 | 0.42 | 0.28 | −0.20 | 0.45* | 0.03 | 0.27 | 0.37 | 0.02 | −0.31 | −0.26 | −0.30 | 0.36 | 0.00 | 0.41 | 0.46* | 0.39 | 1.00 | |
| Bi | 0.79# | −0.29 | −0.05 | 0.80# | 0.71# | 0.36 | 0.81# | 0.33 | 0.71# | 0.55# | 0.09 | 0.30 | 0.18 | 0.33 | −0.16 | 0.05 | 0.80# | 0.61# | 0.78# | 0.38 | 1.00 |
| Note: #, correlation is significant at the 0.01 level (2-tailed). *, correlation is significant at the 0.05 level (2-tailed). | |||||||||||||||||||||
As for Py I, Py II and Py III, the content of S is in the range of 49.09%–54.29% (avg. 51.97%), 50.35%–57.23% (avg. 53.73%) and 50.74%–56.93% (avg. 53.68%), while the content of Fe is in the range of 42.32%–47.48% (avg. 44.32%), 41.54%–49.56% (avg. 45.11%) and 42.28%– 47.85% (avg. 45.42%), respectively. According to the theoretical value of pyrite (S–53.45%, Fe–46.55%), the pyrite in Dongyang deposit are Fe-deficient, and Py I were S-deficient, while the content of S in Py II and Py III were slightly higher than the theoretical value.
The majority of the pyrite are enriched in Te and As, and the values vary a lot (Table 1), and the value of Au is in the range of 0.11×10–6–3.96×10–6 (avg. 1.08×10–6), 0.01×10–6–2.71×10–6 (avg. 0.24×10–6) and 0.01×10–6–4.15×10–6 (avg. 0.22×10–6). W, Mo, Bi and Se have the lowest values, with an average below 20×10–6. As, Co, and Ni substitute significantly in the lattice of pyrite, and the content of As is the most abundant, with an average of 2709×10–6, 4765×10–6, and 2129×10–6, respectively, followed by Co, averaging of 253.50×10–6, 421.42×10–6 and 321.45×10–6, while the value of Ni is relatively low, averaging in 66.89×10–6, 78.90×10–6, and 41.78×10–6, close to the crust value (59×10–6), and varying from the mantle value (2200 ± 500×10–6) (Large RR et al., 2009). The average value of Ag is 200.42×10–6, 18.02×10–6 and 83.42×10–6.
As shown in Table 2, Au and Ag (0.994) have the significant positive correlation in Py I, followed by Te (0.770) and Se (0.539), while negative correlation to Co (–0.658). In Py II, Au only shows positive correlation with arsenic (0.781), while nonsignificant correlation with the other elements (Table 3). For Py III, Au shows significant positive correlation with many elements, such as Ag (0.999), Cu (0.813), Pb (0.997), Ni (0.933), As (0.814), Te (0.991), Mo (0.999), Sb (0.807) and Bi (0.799) (Table 4).
Arsenic shows slight positive correlation with S (0.425) in Py I, and significant positive correlation with Co (0.825), Ni (0.778), Ti (0.952) and W (0.942) (Table 2). In Py II, As only has positive correlation with Au (0.781), while nonsignificant correlation with the other elements (Table 3). In Py III, As shows positive correlation with Au (0.814), Ag (0.807), Cu (0.702), Pb (0.797), Co (0.790), Ni (0.892), Se (0.646), Mo (0.819), Sb (0.603), Te (0.870) and Bi (0.559), while negative correlation with S (–0.484) (Table 4).
In Py I, Te shows positive correlation with Au (0.770), Fe (0.507), Se (0.625) and Ag (0.827), while negative correlation with many other metal elements, such as Cu (–0.669), Pb (–0.602), Zn (–0.525), Mg (–0.592), Cr (–0.622), Mo (–0.640) and Sb (–0.613) (Table 2). Te shows significant correlation with Ag (0.990), Pb (0.657), Co (0.752), Mg (0.916), Mn (0.778), Mo (0.993), Sb (0.787) and Bi (0.783) (Table 3) in Py II. Te in Py III has significant correlation with Au (0.991), Ag (0.991), Cu (0.815), Pb (0.986), Ni (0.967), Co (0.558), As (0.870), Mo (0.993), Sb (0.787) and Bi (0.783) (Table 4).
In summary, Te seems to be the most active chemically elements, as it shows significant correlation with most of the other elements in Py I, Py II and Py III, while Au and As are more active chemically in Py III, indicating different processes during the ore-forming fluid evolution, which will be discussed below.
The ratio of Co/Ni for sedimentary, hydrothermal and volcanic type pyrite is < 1, 1.17–5, and 5–50, respectively, while the volcanic type pyrite is characterized by high value of Co (avg. 480×10–6) and low value of Ni (< 100×10–6) (Bajwah ZU et al., 1987). In this paper, the ratio of Co/Ni for Py I, Py II and Py III is in the range of 0.26–11.46 (avg. 3.79), 0.07–4.93 (avg. 1.88) and 0.42–73.41 (avg. 2.20), respectively, close to that of the hydrothermal type pyrite (Reich M et al., 2005; Qian G et al., 2013; Large RR et al., 2009), and as shown in Fig. 5, most of the samples were plotted in the hydrothermal and mid–low temperature hydrothermal area. Pyrite with low Co/Ni ratio were formed in relative low temperature (Reich M et al., 2005; Tribovillard N et al., 2006; Large RR et al., 2009; Koglin N et al., 2010). Considering the low Co/Ni ratio of the three group pyrite, the authors infer the pyrite may be formed in relative low temperature, which is in accordance to our fluid inclusion result (150°C–190°C, Xu N et al., 2020). The average value of Ni in the three group pyrite is 66.89×10–6, 78.90×10–6 and 41.78×10–6, respectively, close to that of the crust (59×10–6), and much vary from the mantle (2200±500×10–6), indicating crustal origin of those pyrite. The pyrite was most likely formed in post-magmatic fluid (Large RR et al., 2009), agree to the isotope analysis of the ore-forming fluid in the authors’ previous research (Xu N et al., 2020).
The common species in hydrothermal fluids are Au+ (Fleet ME and Knipe SW, 2000) and As3+ in various environment (Pokrovski GS et al., 2002a, 2002b). Arsenic is mainly in two forms in arsenian pyrite: As1− pyrite-[Fe (S, As)2], arsenic substitutes for S in reducing conditions, as As1- (e.g., Simon G et al. 1999a, 1999b ; Blanchard M et al. 2007), and As3+ pyrite-[(Fe, As) S2], arsenic substitutes for Fe in oxidizing conditions, mostly as As3+ (Deditius AP et al. 2008). As1- species exist in pyrite with As concentration from below detection limit (BDL) to 19 % (Deditius AP et al., 2014; Reich M et al., 2005), and As3+ exist with As concentration from BDL to 5.5% (Deditius A et al., 2009a, 2009b; Deditius AP et al., 2008), while As2+ species exist with As concentration as high as 24 % (Qian G et al., 2013). As0 species can also preserve in arsenian pyrite as amorphous As-Fe-S nanoparticle aggregates (Deditius A et al., 2009b; Deditius AP et al., 2014). The precipitation of Au-rich arsenian pyrite is kinetically-favoured and surface-controlled, which may cause the reduction of arsenic (Fleet ME and Mumin AH, 1997; Deditius AP et al., 2014), and the sorption of dissolved As(OH)3 can result in the reduction of As3+ to As1− in the pyrite structure (Pokrovski G et al., 1996), accompanied by the precipitation of FeAsS.
In Py I, the significant positive correlation between As and Co, Ni, Ti, W, Bi (Table 2) may indicate As is mainly in the form of As-complex, which have the ability to adsorb many trace elements (Pokrovski G et al., 1996).
Arsenic in Py II show slight negative correlation with Fe (–0.34), while slight positive correlation with S (0.13), indicating a small number of arsenic substituted for Fe in the lattice, which result in the Fe-deficient feature of this group. Arsenic only show significant positive correlation with Au, suggesting close relationship between Au and the As-bearing fraction. The authors infer gold may be incorporated into As-rich and Fe-deficient surface sites by chemisorption onto As-rich growth surfaces (Fig. 2l; Fleet ME and Mumin AH, 1997).
Arsenic in Py III show significant positive correlation with many metal elements, such as Au, Ag, Cu, Pb, Co, Ni, Se, Mo, Sb, Te and Bi, and significant negative correlation with S, indicating arsenic was more active chemically in this stage, and dominantly substituted for S in the lattice, which suggests the reduction condition in this stage (Deditius AP et al., 2008). As-bearing zones (Fig. 2l) can reflect the ability of arsenic to substitute for S in the lattice of pyrite (Pals DW and Spry PG, 2003; Large RR et al., 2009; Cook NJ et al., 2009a; Keith M et al. 2017), which increase the uptake of gold into the arsenian pyrite crystal structure, referred as “invisible gold”, showing positive correlation between Au and As (Fig. 6; Reich M et al., 2005). Trace elements, including As, Cu, Co, Ni, Pb, Sb and Au, commonly occupy the same growth zones rather than separate growth zones (Deditius AP et al., 2008; Reich M et al., 2005), explaining the significant positive correlation between As and the other elements, resulting in the occurrence of various metal mineral inclusions recognized in the structure of pyrite (Fig. 2). Thus, the authors infer the main elements precipitation, such as As, Cu, Ag, Sb, Pb and Zn, etc, in this stage.
The growth zones most likely reflect abrupt changes in the composition of the pyrite-forming fluid (Reich M et al., 2005). The changes occur in a few processes including reaction with wall rock and minerals precipitation, which produce gradual changes in the hydrothermal systems (Pals DW and Spry PG, 2003; Cook NJ et al., 2009a). Abrupt changes are more likely to occur when fluid separates into different fluids (boils), or when mixes with another fluid. But boiling is essentially a unidirectional process from the compositional standpoint, which deplets the parent fluid in the element and enters vapor phase. It is much less likely to deplete the parent fluid in arsenic (Deditius AP et al., 2014). Although boiling might have played a role in the evolution of the Dongyang gold deposit, it is unlikely to directly control the formation of alternating As-rich zones in pyrite. This leaves fluid mixing as the most likely reason of the observed changes in arsenic contents of the pyrite-forming fluid. The metasomatism of sphalerite to pyrite (Fig. 3b) and tetrahedrite to chalcopyrite (Figs. 2d, f ) indicate the addition of new fluid, and as shown in Fig. 2l, the As-bearing zone in the Py II indicate fluid-mixing during the crystallization of the pyrites.
Arsenic in pyrite can be used to compare the redox environment of ore forming systems from the redox sensitivity of arsenic in vary forms (Keith M et al., 2017; Reich M et al., 2005; Qian G et al., 2013; Deditius AP et al., 2014). Low-sulfur epithermal gold deposits are characterized by reduction conditions (Simmons SF et al., 2005; Deditius AP et al., 2008), coinciding with the formation of As--pyrite. Arsenic occupy the iron site in the pyrite lattice with a high valence (Deditius AP et al., 2008), which occurs in oxidizing hydrothermal systems. The Dongyang gold deposit belong to low-sulfur epithermal deposit, and the mineralogy and quartz fluid inclusion is characteristic by mid-low temperature and neutral-alkaline system (Xu N et al., 2020), varying from oxidizing hydrothermal systems. Thus, arsenic was unlikely to substitute for iron in the structure of pyrite in the reducing environment. The significant substitution of As for S in the arsenian pyrite may imply the more reduced condition in the ore-forming fluid (Keith M et al., 2017; Deditius AP et al., 2014), which was favorable for the precipitation of gold and other metal minerals.
Tellurides and native Te are general Te-bearing minerals (Ciobanu CL et al., 2012), which can also be recognized in As−/Au-rich pyrite (King J et al., 2014) in low-S epithermal gold deposits (Kesler SE et al., 2007; Deditius AP et al., 2014). Te appears in different oxidation states in aqueous solutions (Grundler PV et al., 2013), such as Te2–, Te22–, Te (s), TeO32– and TeO42– (Brugger J et al., 2012), etc., which can be in high concentration (ten to thousands ×10–6) related to many Au deposits (Pals DW and Spry PG, 2003; Ciobanu CL et al., 2006; Sung Y et al., 2009; Yang YC et al., 2011), despite of its low abundance in the crust (5×10–9, Wedepohl KH, 1995). Te is typically enriched in As--pyrite from low-S epithermal systems (Kesler SE et al., 2007), which generally host higher content of Te compared to high-sulfur deposits. Te has high solubility in low-sulfur hydrothermal fluids in neutral to alkaline, low salinity, high temperature and oxygen fugacity (fO2) conditions, (Cook NJ et al., 2009b; Grundler PV et al., 2013; Chouinard A et al., 2005; Gao S et al., 2017), while Te is less soluble in reduced hydrothermal fluids (Grundler PV et al., 2013; Keith M et al., 2017; Goldfarb RJ et al., 2005; Seedorff E et al., 2005; Simmons SF et al., 2005). As, Te and Au preferentially incorporate on the (110) surfaces in cubic crystal pyrite (Cook NJ et al., 2009b; Chouinard A et al., 2005). Arsenic is a key factor to absorb Te in pyrite, and the incorporation of Te is temperature independent but highly sensitive to redox and fO2 changes (Keith M et al., 2017).
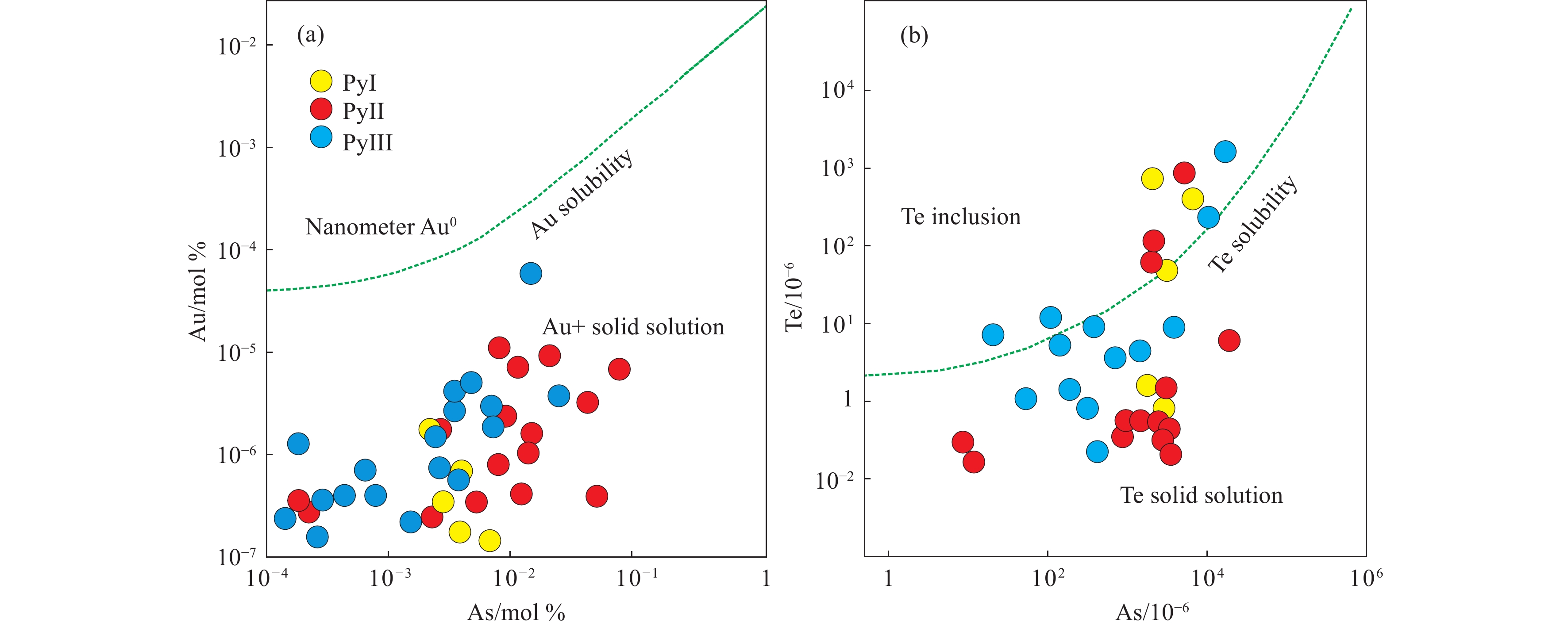
Te in Py I show significant positive correlation with Au, Ag and Se (Table 2; Fig. 7), while strong negative correlation with Zn, Pb, Mg, Cr, Mo and Sb. Te can substitute for S in the lattice of pyrite (Chouinard A et al., 2005; Kesler SE et al., 2007), or be hosted as micron to sub-micron level inclusions (Pals DW and Spry PG, 2003; Cook NJ et al., 2009a; Yang YC et al., 2011). Deditius A et al. (2009a) have recognized Sb-As-Fe-Ni, Sb-Pb-Te and Pb-Bi nanoparticles in arsenian pyrite, and proposed that Te-complexes may promote the incorporation of Au and Ag into pyrite. High Te/Au ratios can reflect the occurrence of telluride inclusions (Keith M et al., 2017). Considering the various Te/Au ratios (4–4211) of the pyrite in this paper, the aulthors propose the occurrence of Te-bearing inclusions. As shown in Fig. 8b, the samples were plotted at Te inclusion and Te solid solution area, which may indicate the existence of Te-complexes and the substitution for S in the pyrite, resulting in the distortion of the pyrite lattice which may provide chance for the substitution of other metal elements.
Tellurium in Py II show significant positive correlation with Ag, Pb, Cr, Mg, Mn, Co and Bi (Table 3; Fig. 7), indicating these elements may precipitate together with the Te-hosted inclusions. As shown in Fig. 2j, the intergrowth between hessite and galena in the fracture of pyrite, can provide evidence to this standpoint. Considered of the relative low content of Au, no obvious correlation between Te and Au may be due to the depletion of Au in Py I stage. The converse correlation of Te with metal elements compared to Py I, may indicate more reducing environment in the fluid (Grundler PV et al., 2013; Keith M et al., 2017).
Tellurium in Py III show more significant positive correlation with more metal elements than Py II, such as Au, Ag, Cu, Pb, Co, Ni, As, Mo, Sb and Bi (Table 4; Fig. 7). The ratio of Te/Au is in the range of 0–3249, and as shown in Fig. 8b, most points were plotted in Te solid solution area, indicating the occurrence of Te inclusions, and most of the Te substituted for S in the crystal structure in this stage. The authors propose fluid mixing in this stage to explain the arsenic-zones in pyrite accompanied by the precipitation of the metal elements (Keith M et al., 2017), as well as the significant positive correlation between these elements.
Thus, Te in Py I may be in form of Te-Au-Ag inclusions and solid solution. Te in Py II may be mostly in form of Te-inclusions, resulting in the positive correlation between Te and the other elements, and along with the fluid evolution, the environment was very likely became more reducing, explaining the change of correlation between Te and the other elements. In Py III, Te may be mainly in form of solid solution, while some occurrence of Te-inclusions also existed, and in this stage, the authors propose fluid mixing to explain the most significant positive correlation with more metal elements compared to Py I and Py II.
Gold generally presents in arsenian pyrite in form of Au1+, Au3+ (Arehart GB et al., 1993; Chouinard A et al., 2005), Au-bearing inclusions, native Au and Au-tellurides (Simon G et al., 1999a, 1999b; Ciobanu CL et al., 2012; Liu JJ et al., 2010, 2015). Gold can substitute for Fe in the distorted octahedral sites (Deditius AP et al., 2014), promoted by the substitution of As1– and As3+, which cause the structural distortion in pyrite (Simon G et al., 1999b; Deditius AP et al., 2008). Au can be also coordinated by two or four S atoms (Simon G et al., 1999a), and cause the dominant lattice distortion in pyrite, because of the relative larger Au+ ionic radius (Simon G et al., 1999a).The oxidized Au and As form in pyrite structure need high fO2 environment (Simmons SF et al., 2005; Su WC et al, 2008). High contents of Au in pyrite is associated with micro-nano sized Au-inclusions and Au-nanoparticles (Deditius AP et al., 2014), and Au value increases along with the size decrease of pyrite in epithermal deposits (Simon G et al., 1999b; Deditius AP et al., 2008). Pressure fluctuation and sulfidation in Au-bearing fluids usually cause the Au precipitation in pyrite fractures (Deditius AP et al., 2014), or along preferential sites (Yang YC et al., 2011; Mills SE et al., 2015; Cook NJ et al., 2013).
According to SEM analysis, Pb, Zn, Cu, Te, Au and Ag were generally detected. The surface scanning in Fig. 9a shows relative homogeneous distributions of Au, Cu and Zn, which vary from the non-uniform distributions of inclusions, suggesting those elements may exist in the pyrite structure. As shown in Fig. 9b, sphalerite inclusion was observed, showing strong signal intensity (Fig. 9e), and the strong signal of Ag and Te in Fig. 9f may indicate the occurrence of Te-Ag inclusions. The weaker signal (about 40 Cps) may indicate a small number of those elements were in form of solid solution in the pyrite (Fig. 9).
Solid solution Au in arsenian pyrite can be promoted by the increase of As concentration and the formation of Fe-deficient surface (Fleet ME and Mumin AH, 1997; Reich M et al., 2005; Su WC et al, 2008), which can be calculated by CAu = 0.02×CAs + 4×10–5 with temperature in the range of about 150°C–250°C, while CAu and CAs represent Au/As concentrations in mol%, respectively. Au is generally in form of solid solution/Au nanoparticles below/above Au solubility limit (Chouinard A et al., 2005; Reich M et al., 2005), and as shown in Fig. 8a, all the points were plotted in the Au+ solid solution area, which may indicate the Au in this deposit is most likely in form of solid solution. Au nanoparticles in arsenian pyrite are stable below 370°C, above which the Au particles coarsening is necessary to avoid melting (Reich M et al., 2006). Pyrite formed < 300°C contain more As (and Au) in solid solution, whereas > 300°C contain less As and mainly host Au as particles (Pokrovski GS et al., 2002a, 2002b; Reich M and Becker U, 2006). The homogenization temperature data from quartz fluid inclusions of Dongyang Au deposit ranges from 130°C–190°C (Xu N et al., 2020), and no evidence appeared for reheating of the pyrite in this deposit, indicating substantial coarsening of the Au nanoparticles did not take place. About 1350×10–6 arsenic was needed for 150×10–6 Au accommodated in the pyrite crystal lattice (Mills SE et al. 2015; Liu JJ et al., 2010, 2015). In this paper, the average value of Au in Py I, Py II and Py III is 1.08×10–6, 0.24×10–6 and 0.22×10–6, while As is 2709×10–6, 4765×10–6, and 2129×10–6, respectively, indicating it is enough for the incorporation of gold into pyrite structure facilitated by arsenic, and this standpoint is also supported by the strong positive correlation between Au and As in Py II (0.781) and Py III (0.814) with low Au concentration (Reich M et al., 2005), whereas Au tend to show correlation with other elements with higher concentration, such as Ag, indicating particle formation which consistent with the observation of particulate electrum (Figs. 2a, c.). The electrum particulates may form in two ways: Firstly, the deformation-caused remobilization of gold can form discrete particles in sulfide (Pokrovski GS et al., 2002a; Tomkins AG, 2007; Cook NJ et al., 2013). The Au remobilization relates to sulfide deformation is either result from solid state diffusion, fluid assisted dissolution, and re-precipitation, or partial melting and elements migration (Mills SE et al., 2015). Secondly, direct precipitation of fracture-filling Au from gold complexes adsorption and reduction (Mills SE, 2013). The remobilization of invisible gold was due to the changes of condition (syn-pyrite), while the precipitation of fracture-filling gold grains is the result of the post incorporation of gold in arsenian pyrite (post-pyrite) (Mills SE et al., 2015; Cook NJ et al., 2013). According to authors’ previous work and research in this paper, the authors propose that Au in Dongyang deposit is mainly in form of invisible Au, thus, the incorporation of gold can be considered as syn-pyrite event. Corrosion textures, referred as “hydrothermal etching”, form through oxidation-dissolution, and require post-pyrite fluid flow through the fractures. As shown in Figs. 2a, c , electrum commonly overgrows these textures, indicating the post-pyrite precipitation. Au locally remobilized from pyrite crystal structures in micro-fractures (Cook NJ et al., 2013), and the concentration of gold along these fractures may be result from the gold remobilization from the pyrite crystal lattice, driven by healing of lattice distortions. The fractures hosting electrum is big (Figs. 2a, c), meaning that they were not healed lattice distortions. Therefore the authors infer the electrum particles can be explained as post-pyrite stage Au-bearing minerals.
An Au-incorporation mechanism of as follow reaction as the As-bearing zones in the pyrites (Simon G et al., 1999b; Liu JJ et al, 2010, 2015):
|
Fe(S,As)2+2Au(HS)0=Fe(S,As)2−Au2S+H2S |
According to this function, Au precipitated during the growth of As-rich rims of pyrite (Fig. 2l), while not on the surface of pre-formed pyrite, characterized by simultaneous precipitation of pyrite and incorporation of Au. In this case, Au delivered as As(HS)0-complexes will be incorporated into As-pyrite as Au+, while reduction of Au+ to Au0 will be favoured when delivered as Au-Cl complexes to the surface of pyrite and other sulfides (Large RR et al., 2009; Liu JJ et al, 2015).
Thus, the Au in this deposit is most likely in form of solid solution (invisible gold), and the incorporation of gold can be considered as syn-pyrite event, while the Au-bearing minerals were the result of post incorporation of gold in arsenian pyrite.
(i) The pyrite in Dongyang gold deposit belong to hydrothermal type, and are Fe-deficient and are enriched in Te and As.
(ii) Arsenic in Py I is mainly in the form of As-complexes, which adsorbed many trace elements. Arsenic was not active chemically in Py II, due to the depletion in the early stage, and gold may be incorporated into As-rich and Fe-deficient surface sites by chemisorption onto As-rich growth surfaces. Arsenic in Py III was the most active chemically, and dominantly substituted for S in the lattice, indicating reducing condition in this stage, which was the main elements precipitation stage.
(iii) Tellurium in Py I was in form of Te-Au-Ag inclusions and solid solution, and mostly in form of Te-inclusions in Py II, while in Py III, Te were mainly in form of solid solution, as well as some occurrence of Te-inclusion.
(vi) Gold in Dongyang deposit is most likely in form of invisible gold, and the incorporation of gold can be considered as syn-pyrite event, while the Au-bearing minerals were the result of post incorporation of gold in arsenian pyrite.
This work was supported by National Natural Science Foundation of China (41872071) and China Geological Survey Project (DD20190006).
|
Arehart GB, Chryssoulis SL, Kesler SE. 1993. Gold and arsenic in iron sulfides from sediment-hosted disseminated gold deposits: Implications for depositional processes. Economic Geology, 88, 171–185. doi: 10.2113/gsecongeo.88.1.171
|
|
Agangi A, Hofmann A, Wohlgemuth-Ueberwasser CC. 2013. Pyrite zoning as a record of mineralization in the Ventersdorp contact reef, Witwatersrand Basin, South Africa. Economic Geology, 108, 1243–1272. doi: 10.2113/econgeo.108.6.1243
|
|
Bajwah ZU, Seccombe PK, Offler P. 1987. Trace element distribution, Co :Ni ratios and genesis of the Big Cadia iron-copper deposit, New South Wales, Australia. Mineral Deposita, 22, 292–300.
|
|
Blanchard M, Alfredsson M, Brodholt J, Wright K, Catlow CRA. 2007. Arsenic incorporation into FeS2 pyrite and its influence on dissolution: ADFT study. Geochimica et Cosmochimica Acta, 71, 624–630. doi: 10.1016/j.gca.2006.09.021
|
|
Brugger J, Etschmann BE, Grundler PV, Liu WH, Testemale D, Pring A. 2012. XAS evidence for the stability of polytellurides in hydrothermal fluids up to 599 degrees C, 800 bar. American Mineralogist, 97, 1519–1522. doi: 10.2138/am.2012.4167
|
|
Chouinard A, Paquette J, William-Jones AE. 2005. Crystallographic controls on trace-element incorporation in auriferous pyrite from Pascua epithermal high sulfidation deposit: Chile-Argentina. Canadian Mineralogist, 43, 951–963. doi: 10.2113/gscanmin.43.3.951
|
|
Ciobanu CL, Cook NJ, Spry PG. 2006. Preface-Special Issue: Telluride and selenide minerals in gold deposits-how and why? Mineralogy and Petrology, 87, 163–169. doi: 10.1007/s00710-006-0133-9
|
|
Ciobanu CL, Cook NJ, Utsunomiya S, Kogagwa M, Green L, Gilbert S, Wade B. 2012. Gold-telluride nanoparticles revealed in arsenic-free pyrite. American Mineralogist, 97, 1515–1518. doi: 10.2138/am.2012.4207
|
|
Cline JS, Hofstra AH, Muntean JL, Tosdal RM, Hickey KA. 2005. Carlin type gold deposits in Nevada: Critical geologic characteristics and viable models. Economic Geology, 100, 451–454.
|
|
Cook NJ, Ciobanu CL, Mao J. 2009a. Textural control on gold distribution in As free pyrite from the Dongping, Huangtuliang and Hougou gold deposits, North China Craton (Hebei Province, China). Chemical Geology, 264, 101–121. doi: 10.1016/j.chemgeo.2009.02.020
|
|
Cook NJ, Ciobanu CL, Spry PG, Voudouris P. 2009b. Understanding gold-(silver)-telluride-(selenide) mineral deposits. Episodes, 32, 249–263. doi: 10.18814/epiiugs/2009/v32i4/002
|
|
Cook NJ, Ciobanu CL, Meria D, Silcock D, Wade B. 2013. Arsenopyrite-pyrite association in an orogenic gold ore: Tracing mineralization history from textures and trace elements. Economic Geology, 108, 1273–1283. doi: 10.2113/econgeo.108.6.1273
|
|
Deditius AP, Utsunomiya S, Renock D, Ewing RC, Ramana CV, Becker U, Kesler SE. 2008. A proposed new form of arsenian pyrite: Composition, nanostructure and geochemical significance. Geochimica et Cosmochimica Acta, 72, 2919–2933. doi: 10.1016/j.gca.2008.03.014
|
|
Deditius A, Utsunomiya S, Ewing RC, Chryssoulis S, Venter D, Kesler SE. 2009a. Decoupled geochemical behavior of As and Cu in hydrothermal systems. Geology, 37, 707–710. doi: 10.1130/G25781A.1
|
|
Deditius A, Utsunomiya S, Ewing RC, Kesler SE. 2009b. Nanoscale “liquid” inclusions of As-Fe-S in arsenian pyrite. American Mineralogist, 94, 394–394.
|
|
Deditius AP, Reich M, Kesler SE, Utsunomiya S, Chryssoulis SL, Walshe J, Ewing RC. 2014. The coupled geochemistry of Au and As in pyrite from hydrothermal ore deposits. Geochimica et Cosmochimica Acta, 140, 644–670. doi: 10.1016/j.gca.2014.05.045
|
|
Einaudi MT, Hedenquist JW, Inan E. 2003. Sulfidation state of fluids in active and extinct hydrothermal systems: Transitions from porphyry to epithermal environments. In: Simmons SF, Graham IJ (Eds.). Volcanic, geothermal and ore-forming fluids: Rulers and witnesses of processes within the Earth Economic Geology, 10, 285–314.
|
|
Fleet ME, Mumin AH. 1997. Gold-bearing arsenian pyrite and marcasite and arsenopyrite from Carlin Trend gold deposits and laboratory synthesis. American Mineralogist, 82, 182–193. doi: 10.2138/am-1997-1-220
|
|
Fleet ME, Knipe SW. 2000. Stability of native gold in H-O-S fluids at 100−400°C and high H2S content. Journal of Solution Chemistry, 29, 1143–1157. doi: 10.1023/A:1005195201175
|
|
Gao S, Xu H, Li SX, Santosh M, Zhang D, Yang L, Quan S. 2017. Hydrothermal alteration and ore-forming fluids associated with gold-tellurium mineralization in the Dongping gold deposit, China. Ore Geology Reviews, 80, 166–184. doi: 10.1016/j.oregeorev.2016.06.023
|
|
Goldfarb RJ, Baker T, Dube B, Groves DI, Hart C, Gosselin P. 2005. Distribution, character, and genesis of gold deposits in metamorphic terranes. Economic Geology, 100, 407–450.
|
|
Grundler PV, Brugger J, Etschmann BE, Helm L, Liu WH, Spry PG, Tian Y, Testemale D, Pring A. 2013. Speciation of aqueous tellurium (IV) in hydrothermal solutions and vapors, and the role of oxidized tellurium species in Te transport and gold deposition. Geochimica et Cosmochimica Acta, 120, 298–325. doi: 10.1016/j.gca.2013.06.009
|
|
Keith M, Smith DJ, Jenkin GRT, Holwell DA, Dye MD. 2017. A review of Te and Se systematics in hydrothermal pyrite from precious metal deposits: Insights into ore-forming processes. Ore Geology Reviews, 126(2), 70–71. doi: 10.1016/j.oregeorev.2017.07.023
|
|
Keith M, Häckel F, Haase KM, Schwarz-Schampera U, Klemd R. 2016a. Trace element systematics of pyrite from submarine hydrothermal vents. Ore Geology Reviews, 72, 728–745. doi: 10.1016/j.oregeorev.2015.07.012
|
|
Keith M, Haase KM, Klemd R, Krumm S, Strauss H. 2016b. Systematic variations of trace element and sulfur isotope compositions in pyrite with stratigraphic depth in the Skouriotissa volcanic-hosted massive sulfide deposit, Troodos ophiolite, Cyprus. Chemical Geology, 423, 7–18. doi: 10.1016/j.chemgeo.2015.12.012
|
|
Kesler SE, Deditius AP, Chryssoulis S. 2007. Geochemistry of Se and Te in arsenian pyrite: New evidence for the role of Se and Te hydrothermal complexes in Carlin and epithermal-type deposits. In: Kojonen KK, Cook NJ, Ojala VJ (Eds.). Au-Ag-Te-Se deposits, Proceedings of the 2007 Field Workshop (Espoo, Finland, August 26-31, 2007). Geological Survey of Finland, 53, 85–95.
|
|
King J, Williams-Jones AE, Van Hinsberg V, Williams-Jones G. 2014. High-sulfidation epithermal pyrite-hosted Au (Ag-Cu) ore formation by condensed magmatic vapors on Sangihe Island, Indonesia. Economic Geology, 109, 1705–1733. doi: 10.2113/econgeo.109.6.1705
|
|
Koglin N, Frimmel HE, Minter WEL. 2010. Trace-element characteristics of different pyrite types in Mesoarchaean to Palaeoproterozoic placer deposits. Mineralium Deposita, 45(3), 259–280. doi: 10.1007/s00126-009-0272-0
|
|
Kouzmanov K, Pettke T, Heinrich CA. 2010. Direct analysis of ore-precipitating fluids: Combined IR microscopy and LA-ICP-MS study of fluid inclusions in opaque ore minerals. Economic Geology, 105, 351–373. doi: 10.2113/gsecongeo.105.2.351
|
|
Large RR, Danyushevsky LV, Hollit C, Maslennikov V, Meffre S, Gilbert SE, Bull S, Scott RJ, Emsbo P, Thomas H, Singh B, Foster J. 2009. Gold and trace element zonation in pyrite using a laser imaging technique: Implications of the timing of gold in orogenic and Carlin-style sediment-hosted deposits. Economic Geology, 104, 635–668. doi: 10.2113/gsecongeo.104.5.635
|
|
Liu JJ, Dai HZ, Zhai DG, Wang JP, Wang YH, Yang LB, Mao GJ, Liu XH, Liao YF, Yu C, Li QZ. 2015. Geological and geochemical characteristics and formation mechanisms of the Zhaishang Carlin-like type gold deposit, western Qinling Mountains, China. Ore Geology Reviews, 64(1), 273–298.
|
|
Liu JJ, Mao GJ, Wu SH, Wang JP, Ma XH, Li LX, Liu GZ, Liao YF, Zheng WJ. 2010. Metallogenic characteristics and formation mechanism of Zhaishang gold deposit, southern Gansu Province. Mineral Deposits, 29(1), 85–100 (in Chinese with English abstract).
|
|
Maslennikov VV, Maslennikov SP, Large RR, Danyushevsky LV. 2009. Study of trace element zonation in vent chimneys from the Silurian Yaman-Kasy volcanic-hosted massive sulfide deposit (Southern Urals, Russia) Using Laser Ablation-Inductively Coupled Plasma Mass Spectrometry (LA-ICPMS). Economic Geology, 104, 1111–1141. doi: 10.2113/gsecongeo.104.8.1111
|
|
Mills SE. 2013. Gold deposit genesis in the Jiaodong Gold District, Northeast China: Mineralogical and geochemical insights into Mesozoic gold in an Archean Craton. (PhD thesis). Monash University.
|
|
Mills SE, Tomkins AG, Weinberg RF, Fan HR. 2015. Implications of pyrite geochemistry for gold mineralisation and remobilisation in the Jiaodong gold district, northeast China. Ore Geology Reviews, 71, 150–168. doi: 10.1016/j.oregeorev.2015.04.022
|
|
Pals DW, Spry PG. 2003. Telluride mineralogy of the low-sulfidation epithermal Emperor gold deposit, Vatukoula, Fiji. Mineralogy and Petrology, 79, 285–307. doi: 10.1007/s00710-003-0013-5
|
|
Pokrovski G, Gout R, Schott J, Zotov A, Harrichoury JC. 1996. Thermodynamic properties and stoichiometry of As (III) hydroxide complexes at hydrothermal conditions. Geochimica et Cosmochimica Acta, 60, 737–749. doi: 10.1016/0016-7037(95)00427-0
|
|
Pokrovski GS, Kara S, Roux J. 2002a. Stability and solubility of arsenopyrite, FeAsS, in crustal fluids. Geochimica et Cosmochimica Acta, 66, 2361–2378. doi: 10.1016/S0016-7037(02)00836-0
|
|
Pokrovski GS, Zakirov IV, Roux J, Testamale D, Hazeman JL, Bychkov AY, Golikova GV. 2002b. Experimental study of arsenic speciation in vapour phase to 500°C: Implications for As transport and fractionation in low-density crustal fluids and volcanic gases. Geochimica et Cosmochimica Acta, 66, 3453–3480. doi: 10.1016/S0016-7037(02)00946-8
|
|
Qian G, Brugger J, Testemale D, Skinner W, Pring A. 2013. Formation of As(II)- pyrite during experimental replacement of magnetite under hydrothermal conditions. Geochimica et Cosmochimica Acta, 100, 1–10. doi: 10.1016/j.gca.2012.09.034
|
|
Reich M, Becker U. 2006. First-principles calculations of the thermodynamic mixing properties of arsenic incorporation into pyrite and marcasite. Chemical Geology, 225, 278–290. doi: 10.1016/j.chemgeo.2005.08.021
|
|
Reich M, Utsunomiya S, Kesler SE, Wang LM, Ewing RC, Becker U. 2006. Thermal behavior of metal nanoparticles in geologic materials. Geology, 34, 1033–1036. doi: 10.1130/G22829A.1
|
|
Reich M, Deditius A, Chryssoulis S, Li JW, Ma CQ, Parada MA, Barra F, Mittermayr F. 2013. Pyrite as a record of hydrothermal fluid evolution in a porphyr copper system: A SIMS/EMPA trace element study. Geochimica et Cosmochimica Acta, 104, 42–62. doi: 10.1016/j.gca.2012.11.006
|
|
Reich M, Kesler SE, Utsunomiya S, Palenik C.S., Chryssoulis, S.L. and Ewing, R. C. 2005. Solubility of gold in arsenian pyrite. Geochimica et Cosmochimica Acta, 69, 2781–2796. doi: 10.1016/j.gca.2005.01.011
|
|
Revan MK, Genç Y, Maslennikov VV, Maslennikov SP, Large RR, Danyushevsky LV. 2014. Mineralogy and trace-element geochemistry of sulfide minerals in hydrothermal chimneys from the Upper-Cretaceous VMS deposits of the Eastern Pontide orogenic belt (NE Turkey). Ore Geology Reviews, 63, 129–149. doi: 10.1016/j.oregeorev.2014.05.006
|
|
Rickard D, Luther GW. 2007. Chemistry of Iron Sulfide. Chemical Reviews, 107, 514–562. doi: 10.1021/cr0503658
|
|
Seedorff E, Dilles J H, Proffett JM, Einaudi MT, Zurcher L, Stavast WJA, Johnson DA, Barton MD. 2005. Porphyry deposits: Characteristics and origin of hypogen features. Economic Geology, 100, 251–298.
|
|
Simon G, Huang H, Penner-Hahn JE, Kesler SE, Kao LS. 1999a. Oxidation state of gold and arsenic in gold-bearing arsenian pyrite. American Mineralogist, 84, 1071–1079. doi: 10.2138/am-1999-7-809
|
|
Simon G, Kesler SE, Chryssoulis S. 1999b. Geochemistry and textures of gold-bearing arsenian pyrite, Twin Creeks, Nevada: Implications for deposition of gold in Carlin-type deposits. Economic Geology, 94, 405–422. doi: 10.2113/gsecongeo.94.3.405
|
|
Simmons SF, White NC, John DA. 2005. Geological characteristics of epithermal precious and base metal deposits. Economic Geology, 100, 485–522.
|
|
Sillitoe RH and Hedenquist JW. 2003. Linkages between volcanic tectonic settings, ore fluid compositions, and epithermal precious metals deposits. In: Simmons SF, Graham IJ (Eds.). Volcanic, geothermal and ore-forming fluids: Rulers and witnesses of processes within the Earth. Economic Geology, 10, 315–343.
|
|
Smith JW, Holwell DA, McDonald I. 2014. Precious and base metal geochemistry and mineralogy of the Grasvally Norite-Pyroxenite-Anorthosite (GNPA) member, northern Bushveld Complex, South Africa: Implications for a multistage emplacement. Mineral Deposita, 49, 667–692. doi: 10.1007/s00126-014-0515-6
|
|
Su WC, Xia B, Zhang HT, Zhang XC, Hu RZ. 2008. Visble gold in arsenian pyrite at the Shuiyindong Carlin-type gold deposit, Guizhou, China: Implications for the environment and processes of ore formation. Ore Geology Reviews, 33, 667–679. doi: 10.1016/j.oregeorev.2007.10.002
|
|
Sung Y, Brugger J, Ciobanu CL, Pring A, Skinner W, Nugus M. 2009. Invisible gold in arsenian pyrite and arsenopyrite from a multistage Archaean gold deposit: Sunrise Dam, Eastern Goldfields Province, Western Australia. Mineral Deposita, 44, 765–791. doi: 10.1007/s00126-009-0244-4
|
|
Tomkins AG. 2007. Three mechanisms of ore re-mobilisation during amphibolite facies metamorphism at the Montauban Zn-Pb-Au-Ag deposit. Mineral Deposita, 42, 627–637. doi: 10.1007/s00126-007-0131-9
|
|
Tribovillard N, Algeo TJ, Lyons T. 2006. Trace metalasas paleoredox and paleoproductivity proxies: An update. Chemical Geology, 232(1 −2), 12–32. doi: 10.1016/j.chemgeo.2006.02.012
|
|
Wedepohl KH. 1995. The composition of the continental-crust. Geochimica et Cosmochimica Acta, 59, 1217–1232. doi: 10.1016/0016-7037(95)00038-2
|
|
Wohlgemuth-Ueberwasser CC, Viljoen F, Petersen S, Vorster C. 2015. Distribution and solubility limits of trace elements in hydrothermal black smoker sulfides: An in-situ LA-ICP-MS study. Geochimica et Cosmochimica Acta, 159, 16–41. doi: 10.1016/j.gca.2015.03.020
|
|
Xu N, Li SR, M Santosh, Tong B. 2017. Petrology, geochemistry and zircon U-Pb geochronology of the Jurassic porphyry dykes in the Dehua gold field, Southeast China: Genesis and geodynamics. Geological Journal, 53, 547–564. doi: 10.1002/gj.2912
|
|
Xu N, Li SR, Wu CL, M Santosh. 2020. Geochemistry and geochronology of the Dongyang gold deposit in southeast China: Constrains to ore genesis. Geological Journal, 55, 425–438. doi: 10.1002/gj.3421
|
|
Yang YC, Liu JJ, Liu XH, Wu SH. 2011. Mode of occurrence of arsenic and its influence on the precipitation of gold in the Jinlongshan gold deposit, southern Qinling. Geology in China, 38(3), 701–715 (in Chinese with English abstract).
|
| NO. | Stage | S/% | Fe/% | Cu | Zn | Pb | Co | Ni | As | Se | Mg | Al | Mn |
| 117-2 | Py I | 52.78 | 45.68 | 52.81 | 3.08 | 214.96 | 630.02 | 150.03 | 5160.69 | 5.83 | 28.70 | 309.21 | 20.16 |
| 240-1 | Py I | 54.29 | 39.05 | 253.62 | 119.85 | 499.34 | 80.67 | 17.82 | 2828.20 | 0.00 | 6918.19 | 20681.55 | 308.34 |
| 240-3 | Py I | 49.09 | 42.32 | 46.54 | 47.67 | 726.41 | 265.47 | 23.17 | 1681.40 | 0.00 | 4871.61 | 8165.10 | 5740.33 |
| 340-1 | Py I | 51.15 | 47.48 | 169.77 | 3.15 | 66.12 | 276.34 | 85.30 | 2316.20 | 9.71 | 25.66 | 491.92 | 8.73 |
| 3128-2 | Py I | 52.55 | 47.09 | 3.85 | 0.52 | 23.82 | 14.98 | 58.15 | 1556.58 | 11.54 | 0.47 | 1.21 | 0.15 |
| 13-2 | Py II | 53.36 | 44.97 | 6.56 | 4.94 | 21.56 | 2.93 | 2.05 | 13040.62 | 2.95 | 27.71 | 404.55 | 2.66 |
| 13-21 | Py II | 51.77 | 47.85 | 5.05 | 1.24 | 16.97 | 12.55 | 5.19 | 3220.99 | 4.30 | 6.23 | 44.08 | 1.88 |
| 13-24 | Py II | 50.89 | 48.33 | 11.20 | 2.57 | 23.25 | 12.25 | 5.44 | 1605.86 | 0.00 | 23.96 | 213.27 | 9.49 |
| 117-3 | Py II | 53.72 | 45.68 | 8.89 | 2.60 | 22.82 | 86.51 | 24.56 | 1990.04 | 2.55 | 3.70 | 333.95 | 1.40 |
| 125-1 | Py II | 54.13 | 41.54 | 418.89 | 36675.19 | 319.32 | 31.68 | 6.43 | 3175.61 | 0.00 | 141.83 | 514.67 | 241.28 |
| 125-2 | Py II | 55.07 | 44.42 | 18.32 | 0.00 | 69.54 | 217.15 | 2.20 | 2720.92 | 0.49 | 12.10 | 430.78 | 11.79 |
| 125-22 | Py II | 54.89 | 44.79 | 24.01 | 2.70 | 65.31 | 4.41 | 4.98 | 960.04 | 0.56 | 21.29 | 486.88 | 48.83 |
| 179-1 | Py II | 53.87 | 45.44 | 35.58 | 1.13 | 171.04 | 0.21 | 0.38 | 862.34 | 0.00 | 65.84 | 1097.15 | 18.31 |
| 22-1 | Py II | 52.17 | 47.37 | 15.65 | 0.73 | 27.22 | 1108.32 | 3113.78 | 8.54 | 0.59 | 0.12 | 0.31 | 0.38 |
| 22-12 | Py II | 52.81 | 46.57 | 80.37 | 0.79 | 24.90 | 1446.51 | 4240.78 | 9.74 | 0.00 | 1.43 | 9.24 | 0.23 |
| 310-3 | Py II | 50.36 | 49.56 | 8.01 | 0.66 | 29.22 | 4.24 | 1.07 | 330.73 | 0.00 | 4.18 | 3.81 | 1.72 |
| 360-1 | Py II | 52.99 | 45.20 | 37.05 | 4.59 | 7.46 | 3.40 | 7.45 | 17227.57 | 8.38 | 11.85 | 165.11 | 1.15 |
| 360-2 | Py II | 55.32 | 42.28 | 59.01 | 17.30 | 177.71 | 781.21 | 201.18 | 1966.23 | 11.51 | 410.73 | 3692.21 | 131.23 |
| 3127-3 | Py II | 51.54 | 46.92 | 22.86 | 10.66 | 425.29 | 2642.24 | 2156.33 | 4720.39 | 4.36 | 879.64 | 20.11 | 347.58 |
| 3128-11 | Py II | 55.31 | 41.83 | 30.47 | 2.16 | 61.53 | 18.27 | 58.95 | 28097.12 | 3.13 | 5.76 | 18.59 | 0.39 |
| 3128-12 | Py II | 57.23 | 42.29 | 31.33 | 5.99 | 48.02 | 0.57 | 8.40 | 2550.44 | 3.91 | 48.53 | 471.02 | 2.75 |
| 614-11 | Py II | 56.05 | 43.10 | 89.30 | 1.60 | 99.31 | 1163.01 | 259.55 | 2868.70 | 0.00 | 10.82 | 507.95 | 4.30 |
| 614-12 | Py II | 55.62 | 43.86 | 360.68 | 1331.71 | 262.02 | 50.10 | 38.84 | 406.05 | 11.52 | 11.87 | 41.50 | 10.47 |
| 13-11 | Py III | 53.36 | 46.15 | 20.71 | 4.62 | 124.93 | 163.38 | 9.23 | 1267.27 | 0.00 | 76.52 | 369.37 | 36.95 |
| 13-12 | Py III | 53.11 | 46.35 | 15.51 | 0.99 | 88.77 | 42.47 | 3.08 | 394.47 | 0.69 | 10.19 | 682.65 | 32.07 |
| 13-22 | Py III | 51.77 | 47.85 | 5.05 | 1.24 | 16.97 | 12.55 | 5.19 | 3220.99 | 4.30 | 6.23 | 44.08 | 1.88 |
| 13-23 | Py III | 52.67 | 47.02 | 5.88 | 0.00 | 17.78 | 15.30 | 6.60 | 1495.93 | 1.39 | 20.76 | 89.73 | 9.02 |
| 181-1 | Py III | 56.93 | 42.66 | 12.15 | 3.11 | 79.69 | 268.00 | 3.65 | 1598.41 | 0.00 | 94.84 | 274.96 | 44.53 |
| 27-11 | Py III | 53.72 | 43.56 | 7.93 | 0.00 | 14.70 | 153.84 | 113.03 | 664.25 | 0.38 | 4988.44 | 27.43 | 2240.83 |
| 27-12 | Py III | 54.09 | 45.17 | 18.58 | 8.94 | 38.40 | 157.84 | 93.67 | 336.19 | 0.58 | 683.06 | 757.85 | 337.57 |
| 27-13 | Py III | 53.09 | 43.29 | 27.25 | 26.89 | 65.26 | 85.06 | 77.18 | 107.31 | 0.00 | 4249.11 | 3404.40 | 2761.94 |
| 214-1 | Py III | 56.08 | 43.79 | 2.44 | 0.83 | 7.71 | 21.56 | 34.17 | 52.09 | 0.26 | 20.99 | 252.63 | 1.39 |
| 214-21 | Py III | 54.83 | 44.88 | 16.40 | 1.33 | 53.65 | 81.01 | 87.85 | 132.52 | 2.05 | 8.10 | 455.44 | 0.74 |
| 214-22 | Py III | 55.61 | 43.80 | 21.63 | 0.00 | 67.73 | 156.11 | 208.21 | 190.55 | 0.00 | 10.65 | 1012.42 | 1.63 |
| 214-23 | Py III | 53.00 | 46.86 | 12.42 | 0.86 | 39.56 | 140.67 | 166.13 | 118.08 | 3.63 | 14.35 | 216.99 | 1.72 |
| 230-1 | Py III | 55.72 | 43.81 | 30.44 | 0.34 | 131.96 | 88.97 | 11.85 | 3536.04 | 1.54 | 11.76 | 65.40 | 16.50 |
| 230-3 | Py III | 55.48 | 44.21 | 14.58 | 1.44 | 47.73 | 5.46 | 5.01 | 2092.6 | 2.64 | 20.82 | 54.50 | 2.23 |
| 34-11 | Py III | 51.22 | 48.63 | 5.37 | 3.32 | 72.82 | 139.02 | 14.48 | 293.53 | 1.87 | 11.40 | 149.28 | 11.09 |
| 360-2 | Py III | 55.32 | 42.28 | 59.01 | 17.3 | 177.71 | 781.21 | 201.18 | 1966.23 | 11.51 | 410.73 | 3692.21 | 131.23 |
| 382-22 | Py III | 54.14 | 44.75 | 104.49 | 7.54 | 56.94 | 881.75 | 217.24 | 1167.76 | 1.62 | 139.88 | 2351.25 | 18.74 |
| 3127-2 | Py III | 53.59 | 46.33 | 0.27 | 0.64 | 0.67 | 100.37 | 241.13 | 19.20 | 5.75 | 1.09 | 16.47 | 1.21 |
| 3128-21 | Py III | 50.56 | 48.03 | 28.40 | 0.24 | 18.09 | 2104.00 | 650.62 | 10398.53 | 14.03 | 0.00 | 0.00 | 0.10 |
| 3128-22 | Py III | 50.74 | 46.85 | 165.86 | 1.72 | 2589.79 | 1351.22 | 1844.08 | 15522.4 | 7.90 | 9.10 | 62.13 | 1.54 |
| 614-3 | Py III | 52.19 | 47.51 | 6.98 | 0.00 | 37.15 | 0.75 | 0.16 | 130.46 | 0.35 | 35.29 | 247.98 | 15.22 |
| Au | S | Fe | Ag | Cu | Zn | Pb | Co | Ni | As | Se | Mg | Al | Mn | Ti | Cr | Mo | Sb | Te | W | Bi | |
| Au | 1.00 | ||||||||||||||||||||
| S | 0.23 | 1.00 | |||||||||||||||||||
| Fe | 0.29 | −0.23 | 1.00 | ||||||||||||||||||
| Ag | 0.99# | 0.25 | 0.37 | 1.00 | |||||||||||||||||
| Cu | −0.45 | 0.44 | −0.55 | −0.50 | 1.00 | ||||||||||||||||
| Zn | −0.22 | 0.35 | −0.96# | −0.30 | 0.70 | 1.00 | |||||||||||||||
| Pb | −0.44 | −0.34 | −0.82 | −0.51 | 0.18 | 0.68 | 1.00 | ||||||||||||||
| Co | −0.66 | −0.15 | 0.24 | −0.58 | −0.21 | −0.39 | 0.00 | 1.00 | |||||||||||||
| Ni | −0.22 | 0.14 | 0.64 | −0.11 | −0.31 | −0.69 | −0.58 | 0.81 | 1.00 | ||||||||||||
| As | −0.49 | 0.42 | 0.02 | −0.41 | 0.07 | −0.10 | −0.12 | 0.83 | 0.78 | 1.00 | |||||||||||
| Se | 0.54 | 0.08 | 0.91* | 0.60 | −0.38 | −0.81 | −0.95* | −0.07 | 0.49 | −0.09 | 1.00 | ||||||||||
| Mg | −0.26 | 0.08 | −0.97# | −0.35 | 0.54 | 0.95* | 0.85 | −0.36 | −0.77 | −0.21 | −0.90* | 1.00 | |||||||||
| Al | −0.22 | 0.35 | −0.96# | −0.30 | 0.71 | 1.00# | 0.68 | −0.39 | −0.69 | −0.10 | −0.80 | 0.95* | 1.00 | ||||||||
| Mn | −0.25 | −0.80 | −0.36 | −0.31 | −0.28 | 0.19 | 0.81 | 0.01 | −0.48 | −0.39 | −0.60 | 0.47 | 0.19 | 1.00 | |||||||
| Ti | −0.36 | 0.26 | 0.16 | −0.27 | −0.24 | −0.29 | −0.14 | 0.88 | 0.84 | 0.95* | 0.00 | −0.35 | −0.30 | −0.27 | 1.00 | ||||||
| Cr | −0.40 | −0.69 | −0.55 | −0.46 | −0.03 | 0.40 | 0.91* | −0.02 | −0.57 | −0.35 | −0.76 | 0.65 | 0.40 | 0.96# | −0.30 | 1.00 | |||||
| Mo | −0.30 | 0.37 | −0.87 | −0.38 | 0.87 | 0.95* | 0.54 | −0.40 | −0.65 | −0.12 | −0.69 | 0.87 | 0.95* | 0.05 | −0.37 | 0.29 | 1.00 | ||||
| Sb | −0.30 | 0.46 | −0.79 | −0.37 | 0.92* | 0.91* | 0.41 | −0.39 | −0.57 | −0.07 | −0.59 | 0.79 | 0.91* | −0.09 | −0.34 | 0.16 | 0.98# | 1.00 | |||
| Te | 0.77 | 0.34 | 0.51 | 0.83 | −0.67 | −0.52 | −0.60 | −0.04 | 0.40 | 0.13 | 0.62 | −0.59 | −0.53 | −0.44 | 0.31 | −0.62 | −0.64 | −0.61 | 1.00 | ||
| W | −0.36 | 0.22 | 0.21 | −0.27 | −0.27 | −0.35 | −0.17 | 0.89* | 0.87 | 0.94* | 0.04 | −0.40 | −0.35 | −0.26 | 0.99# | −0.30 | −0.42 | −0.38 | 0.31 | 1.00 | |
| Bi | −0.34 | 0.28 | 0.16 | −0.25 | −0.24 | −0.29 | −0.15 | 0.86 | 0.84 | 0.95* | 0.00 | −0.35 | −0.29 | −0.28 | 1.00# | −0.31 | −0.37 | −0.34 | 0.33 | 0.99# | 1.00 |
| Note: #, correlation is significant at the 0.01 level (2-tailed). *, correlation is significant at the 0.05 level (2-tailed). | |||||||||||||||||||||
| Au | S | Fe | Ag | Cu | Zn | Pb | Co | Ni | As | Se | Mg | Al | Mn | Ti | Cr | Mo | Sb | Te | W | Bi | |
| Au | 1.00 | ||||||||||||||||||||
| S | 0.28 | 1.00 | |||||||||||||||||||
| Fe | −0.40 | −0.88# | 1.00 | ||||||||||||||||||
| Ag | 0.02 | −0.24 | 0.14 | 1.00 | |||||||||||||||||
| Cu | −0.09 | 0.29 | −0.46 | −0.06 | 1.00 | ||||||||||||||||
| Zn | −0.08 | 0.06 | −0.38 | −0.05 | 0.75# | 1.00 | |||||||||||||||
| Pb | −0.03 | 0.10 | −0.29 | 0.70# | 0.58* | 0.46 | 1.00 | ||||||||||||||
| Co | −0.04 | −0.19 | 0.16 | 0.77# | −0.10 | −0.14 | 0.46 | 1.00 | |||||||||||||
| Ni | −0.10 | −0.30 | 0.31 | 0.30 | −0.09 | −0.11 | 0.05 | 0.73# | 1.00 | ||||||||||||
| As | 0.78# | 0.13 | −0.34 | −0.02 | −0.15 | −0.06 | −0.16 | −0.19 | −0.22 | 1.00 | |||||||||||
| Se | 0.01 | 0.27 | −0.30 | 0.15 | 0.23 | −0.18 | 0.25 | −0.01 | −0.20 | 0.19 | 1.00 | ||||||||||
| Mg | −0.02 | −0.15 | −0.02 | 0.92# | −0.01 | 0.05 | 0.75# | 0.71# | 0.22 | −0.06 | 0.28 | 1.00 | |||||||||
| Al | −0.13 | 0.34 | −0.40 | −0.05 | −0.01 | 0.01 | 0.18 | 0.01 | −0.21 | −0.15 | 0.42 | 0.30 | 1.00 | ||||||||
| Mn | −0.06 | −0.12 | −0.16 | 0.80# | 0.33 | 0.49* | 0.86# | 0.56* | 0.15 | −0.09 | 0.11 | 0.88# | 0.20 | 1.00 | |||||||
| Ti | −0.11 | 0.25 | −0.14 | −0.07 | 0.60# | 0.01 | 0.31 | −0.20 | −0.18 | −0.15 | 0.55* | −0.12 | −0.09 | −0.10 | 1.00 | ||||||
| Cr | −0.01 | 0.25 | −0.30 | 0.54* | 0.13 | −0.10 | 0.57* | 0.37 | 0.00 | −0.06 | 0.75# | 0.73# | 0.60# | 0.54* | 0.25 | 1.00 | |||||
| Mo | −0.12 | 0.06 | −0.39 | −0.07 | 0.70# | 0.97# | 0.43 | −0.17 | −0.17 | −0.07 | −0.14 | 0.08 | 0.14 | 0.50* | −0.02 | −0.05 | 1.00 | ||||
| Sb | −0.17 | 0.33 | −0.37 | 0.22 | 0.63# | 0.43 | 0.74# | 0.12 | −0.14 | −0.30 | 0.04 | 0.26 | 0.17 | 0.41 | 0.39 | 0.21 | 0.38 | 1.00 | |||
| Te | 0.01 | −0.27 | 0.17 | 0.99# | −0.07 | 0.65# | 0.75# | 0.30 | −0.02 | 0.13 | 0.91# | −0.05 | 0.77# | −0.09 | 0.52* | −0.10 | 0.15 | 1.00 | |||
| W | −0.13 | 0.27 | −0.24 | −0.07 | 0.78# | 0.26 | 0.42 | −0.20 | −0.18 | −0.18 | 0.48* | −0.10 | −0.10 | 0.04 | 0.96# | 0.22 | 0.23 | 0.46 | −0.10 | 1.00 | |
| Bi | −0.01 | −0.06 | 0.05 | 0.75# | −0.08 | −0.14 | 0.52* | 0.56* | 0.13 | −0.11 | 0.22 | 0.70# | 0.08 | 0.54* | 0.11 | 0.49* | −0.16 | 0.16 | 0.81# | 0.06 | 1.00 |
| Note: #, correlation is significant at the 0.01 level (2-tailed). *, correlation is significant at the 0.05 level (2-tailed). | |||||||||||||||||||||
| Au | S | Fe | Ag | Cu | Zn | Pb | Co | Ni | As | Se | Mg | Al | Mn | Ti | Cr | Mo | Sb | Te | W | Bi | |
| Au | 1.00 | ||||||||||||||||||||
| S | −0.37 | 1.00 | |||||||||||||||||||
| Fe | 0.17 | −0.85# | 1.00 | ||||||||||||||||||
| Ag | 1.0# | −0.38 | 0.17 | 1.00 | |||||||||||||||||
| Cu | 0.81# | −0.22 | −0.04 | 0.81# | 1.00 | ||||||||||||||||
| Zn | −0.07 | 0.07 | −0.43 | −0.07 | 0.19 | 1.00 | |||||||||||||||
| Pb | 0.99# | −0.35 | 0.14 | 0.99# | 0.82# | −0.04 | 1.00 | ||||||||||||||
| Co | 0.45* | −0.41 | 0.18 | 0.45* | 0.61# | 0.04 | 0.44* | 1.00 | |||||||||||||
| Ni | 0.93# | −0.45* | 0.21 | 0.93# | 0.82# | −0.06 | 0.92# | 0.70# | 1.00 | ||||||||||||
| As | 0.81# | −0.48* | 0.28 | 0.80# | 0.70# | −0.15 | 0.79# | 0.79# | 0.89# | 1.00 | |||||||||||
| Se | 0.31 | −0.39 | 0.22 | 0.31 | 0.36 | 0.05 | 0.30 | 0.79# | 0.54* | 0.64# | 1.00 | ||||||||||
| Mg | −0.09 | −0.02 | −0.38 | −0.09 | −0.08 | 0.47* | −0.09 | −0.12 | −0.09 | −0.17 | −0.22 | 1.00 | |||||||||
| Al | −0.13 | 0.20 | −0.51* | −0.12 | 0.29 | 0.88# | −0.09 | 0.11 | −0.08 | −0.20 | 0.11 | 0.32 | 1.00 | ||||||||
| Mn | −0.09 | −0.04 | −0.37 | −0.08 | −0.08 | 0.57# | −0.09 | −0.13 | −0.09 | −0.17 | −0.23 | 0.98# | 0.39 | 1.00 | |||||||
| Ti | −0.21 | 0.19 | −0.07 | −0.20 | −0.22 | −0.04 | −0.19 | −0.30 | −0.25 | −0.28 | −0.13 | −0.05 | −0.04 | −0.04 | 1.00 | ||||||
| Cr | 0.02 | 0.22 | −0.39 | 0.01 | 0.37 | 0.46* | 0.06 | 0.29 | 0.06 | 0.04 | 0.44* | −0.08 | 0.70# | −0.11 | −0.06 | 1.00 | |||||
| Mo | 0.99# | −0.38 | 0.16 | 0.99# | 0.81# | −0.05 | 0.99# | 0.47* | 0.94# | 0.81# | 0.34 | −0.08 | −0.10 | −0.08 | −0.20 | 0.04 | 1.00 | ||||
| Sb | 0.80# | −0.06 | −0.07 | 0.80# | 0.69# | −0.08 | 0.82# | 0.27 | 0.75# | 0.60# | 0.15 | −0.12 | −0.05 | −0.11 | 0.07 | 0.01 | 0.80# | 1.00 | |||
| Te | 0.99# | −0.42 | 0.20 | 0.99# | 0.81# | −0.07 | 0.98# | 0.55# | 0.96# | 0.87# | 0.41 | −0.09 | −0.12 | −0.09 | −0.23 | 0.03 | 0.99# | 0.78# | 1.00 | ||
| W | 0.42 | 0.10 | −0.02 | 0.42 | 0.28 | −0.20 | 0.45* | 0.03 | 0.27 | 0.37 | 0.02 | −0.31 | −0.26 | −0.30 | 0.36 | 0.00 | 0.41 | 0.46* | 0.39 | 1.00 | |
| Bi | 0.79# | −0.29 | −0.05 | 0.80# | 0.71# | 0.36 | 0.81# | 0.33 | 0.71# | 0.55# | 0.09 | 0.30 | 0.18 | 0.33 | −0.16 | 0.05 | 0.80# | 0.61# | 0.78# | 0.38 | 1.00 |
| Note: #, correlation is significant at the 0.01 level (2-tailed). *, correlation is significant at the 0.05 level (2-tailed). | |||||||||||||||||||||