| Citation: Zhang Zhen-fang, Zhang Wei-bo, Zhang Zhen-guo, Chen Xiu-fa. 2025. Nickel extraction from nickel laterites: Processes, resources, environment and cost. China Geology, 8(1), 187‒213. doi: 10.31035/cg2024124. |
Nickel, as an important industrial metal, is widely used in steel, alloys, electroplating, batteries, magnetic materials and other fields due to its excellent mechanical strength, ductility, magnetism and high chemical stability. Since the beginning of the 21st century, its applications have gradually expanded from traditional industries such as stainless steel, alloys, electroplating and catalysts to emerging fields like new energy and new materials. In recent years, with the development of the new energy industry, nickel has been used in ternary power battery materials and has become a crucial energy metal. It has been listed as a strategic mineral by many countries and is receiving more and more attention worldwide (Andersson P, 2020; Brown T, 2018).
The statistics from the International Energy Agency has showed that the proportion of nickel used in the energy industry increased from 6% of 2017 to 16% of 2022, which is the primary factor driving the growth in global demand for primary nickel. It is predicted that by 2050, under the announced pledges scenario, global primary nickel demand will reach 6.5×106 t, of which the clean energy sector will reach 3.88×106 t, accounting for 60%; under the net zero emission scenario, these figures are 6.19×106 t and 3.76 ×106 t respectively. According to statistics from S&P Global, the global primary nickel production in 2022 was 3.03×106 t. Based on the global production forecast from nickel mines in production and in development, the average annual growth rate of primary nickel production over the next five years is expected to be approximately 7.6%, with global primary nickel supply reaching about 4.37×106 t by 2027.
Fraser J (2021) predicts that the global primary nickel supply could be in short around 2028, with a structural deficit of about 0.977×106 t by 2040, mainly in the clean energy sector. As a result, battery-grade nickel has been included in the European Union’s fifth edition of the critical raw materials list in 2023 (European Commission et al., 2023). Although Indonesia, the largest supplier of primary nickel, has made progress in transitioning nickel pig iron (NPI) to nickel matte, and several high-pressure acid leaching plants are under construction to meet the demand for primary nickel in the clean energy sector (Heijlen W and Duhayon C, 2024), the transition faces potential challenges such as higher energy consumption, while high-pressure acid leaching has environmental concerns. There is an urgent need to further improve nickel extraction technology to meet the demand of clean energy transition and comprehensive resource utilization (IEA, 2023).
Most of the world’s terrestrial nickel resources are hosted in lateritic weathering crusts (55%) and magmatic sulfides (28%) which are commercially mined. The deep-sea manganese nodules (17%) are still on the way (Guo YS et al., 2013). The distribution of nickel resources in the world is uneven, mainly concentrated in Indonesia, Australia, Canada, Russia, New Caledonia, Brazil, the Philippines, Cuba, China and South Africa (Table 1). Most lateritic nickel resources occur within a band about 22 degrees of latitude either side of the equator, such as Indonesia and the Philippines in Southeast Asia, New Caledonia, eastern Australia and Papua New Guinea in Oceania, and Brazil, Cuba, Colombia and Dominican in the Caribbean region of Central America (Yang XS et al., 2013).
| Country | Reserves/Mt | Global share/% | Resources (including reserves) /Mt) | Global share/% |
| Indonesia | 21 | 21 | 71 | 19 |
| Australia | 21 | 21 | 40 | 11 |
| Brazil | 16 | 16 | 19 | 5 |
| Russia | 7.5 | 7.5 | 36 | 10 |
| New Caledonia | 7.1 | 7.1 | 31 | 8 |
| The Philippines | 4.8 | 4.8 | 31 | 8 |
| Canada | 2.2 | 2.2 | 39 | 11 |
| China* | 2.1 | 2.1 | 9 | 2 |
| Others | 20 | 20 | 89 | 24 |
| Total | >100 | 100 | 365 | 100 |
| Data source: Reserve data come from USGS Mineral Commodity Summaries 2023, resource data come from S&P Global Market Intellgence Mineral Database (data as of May 2023). *According to the China Mineral Resources Report (2022), China's nickel reserves are 4.22×106 t. | ||||
According to incomplete statistics from the S&P Mineral Database, there were 266 nickel deposits globally with nickel resources (including reserves) greater than or equal to 100000 t in 2023. Among these, laterite nickel ore accounts for approximately 63% of the total resources (Fig.1).
The Asia-Pacific region ranks first with 131 deposits and 191×106 t of nickel metal resources, accounting for 53% of the total resources. This is followed by Europe with 36 deposits, accounting for 14% of the total resources; Latin America and the Caribbean with 29 deposits, accounting for 12%; Canada with 25 deposits, accounting for 10%; Africa with 36 deposits, accounting for 8%; and the United States with 9 deposits, accounting for 3%. In the Asia-Pacific region, Indonesia has 19 deposits, accounting for 37% of the regional total resources; Australia has 55 deposits, accounting for 20% and the Philippines and New Caledonia have 29 and 6 deposits respectively, each accounting for 15% of the regional total resources (Fig. 2; Zhang ZF et al. 2022).
With the continuous increase in global nickel demand and the gradual depletion of sulfide nickel ore resources, the production share of laterite nickel ore has risen from 20% in the 1950s to about 70% today, gradually becoming the main source of nickel (Zhang ZF et al., 2022). This shift is attributed to the ongoing development and improvement of laterite nickel ore processing technologies. The first technological revolution occurred with the demand for nickel in stainless steel soaring, leading to the adoption of the Rotary Kiln-Electric Furnace (RKEF) process. This allowed the abundant laterite nickel ores to enter the stainless steel supply chain, rapidly increasing ferronickel production and significantly reducing the production costs of the stainless steel industry, which in turn promoted large-scale development of stainless steel. The second technological revolution occurred with the sharp rise in demand for nickel in power batteries, leading to the use of the High-Pressure Acid Leaching (HPAL) process, which once again compensated for the structural shortage of nickel sulfate caused by insufficient sulfide nickel ore supply. However, no single process is perfect, and with the advancement of technology and changes in demand, nickel extraction technologies for laterite nickel ore are still being continuously developed and improved. This article aims to review the main nickel extraction technologies for laterite nickel ore, discuss the current status and characteristics of these technologies, and compare industrialized production processes in terms of resources, yield, environment and costs, exploring the comprehensive utilization direction of laterite nickel ore.
S&P Global Commodity Insights has identified 82 major nickel discoveries made from 1990 to 2022, containing 147 ×106 t of nickel in oxide and sulfide deposits and the former accounting for 68%. These deposits are primarily located in Indonesia (47.57×106 t), Australia (19.87×106 t), the Philippines (9.43×106 t), Cuba (7.65×106 t), and Brazil (7.18×106 t). Currently mining deposits include Weda Bay in Indonesia, Murrin Murrin in Australia, Barro Alto in Brazil, Koniambo in New Caledonia, and Cagdianao in the Philippines.
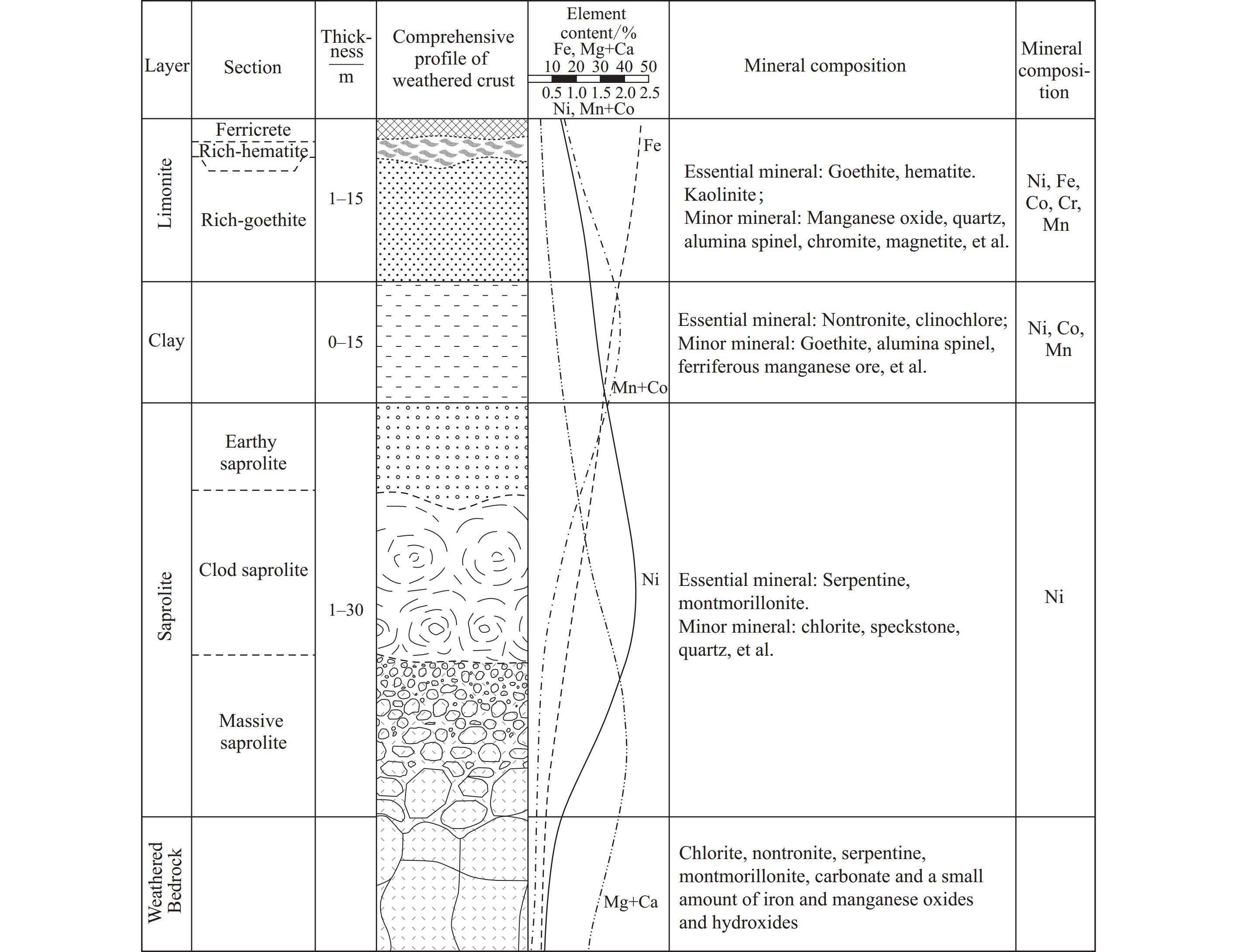
Nickel laterites are supergene ore bodies formed by the enrichment of dispersed nickel through intense chemical weathering of ultramafic rocks such as dunite, peridotite, and serpentine. In this process, nickel is released from silicate minerals like olivine and enstatite and replaces iron or magnesium in the crystal lattice, forming new nickel-bearing secondary minerals (Wang CY and Ma BZ, 2020). All nickel laterites follow a similar weathering profile and lateritic ore bodies can be divided into three subtypes: Oxide laterites (Limonite), clay laterites (Nontronite) and silicate laterites (Saprolite) (Fig. 3). Oxide laterites are mostly constituted of the limonite layer. The primary nickel-bearing minerals in limonite are goethite (FeO‧OH) and hematite (Fe2O3), with gangue minerals such as spinel, talc, and amphibole, accounting for 61% of the world's laterite nickel reserve. Clay laterites are formed in colder and drier climates. The primary nickel-bearing minerals are garnierite and saponite, accounting for 7% of the world's laterite nickel reserves. Silicate laterites are formed as a result of the slow tectonic uplift with a low water table in the profile. The primary nickel-bearing minerals in saprolite are serpentine, montmorillonite, and chlorite, accounting for 32% of the world's laterite nickel ore reserves (König U, 2021; Stanković S et al., 2020).
The contents of nickel and other associated elements (Fe, Co, Si, Mg, etc.) vary significantly across different types of laterite nickel ores (Table 2; Tian QH et al., 2023; Shi ZY et al., 2019). Additionally, the laterite nickel ore properties vary significantly across different regions. Wet-type laterite nickel ores from regions near the equator, such as Indonesia and New Caledonia, have relatively higher nickel grades, lower clay mineral content, simpler composition, and are easier to process and extract, with thin overburden layers that are easier to mine. In contrast, dry-type laterite nickel ores from regions farther from the equator, such as Western Australia, have relatively lower nickel grades, higher clay mineral content, more complex and variable composition, making them harder to process and with a larger stripping ratio (Zevgolis EN and Daskalakis KA, 2022).
| Ore type | Mass fraction/% | Characteristics | Extraction process | |||||
| Ni | TFe | MgO | SiO2 | Co | Cr2O3 | |||
| Limonite | 0.8‒1.4 | 36‒50 | 0.5‒5.0 | 0‒10 | 0.1‒0.2 | 2‒5 | Low Ni & Mg, high Fe | Hydrometallurgy |
| Nontronite | 1.2‒1.8 | 25‒40 | 5‒15 | 10‒30 | 0.02‒0.1 | 1‒2 | Pyrometallurgy/ Hydrometallurgy |
|
| Saprolite | 1.4‒3.0 | 10‒25 | 15‒35 | 30‒50 | 0.02‒0.1 | 1‒2 | High Ni, Mg &Si, low Fe | Pyrometallurgy |
In summary, compared with sulfide nickel ore, laterite nickel ore has low nickel grade, more complex nickel occurrence and process mineralogy, and great composition fluctuations. It is difficult to beneficiate and recover by simple physical sorting methods, so metallurgical methods are usually used to obtain higher-grade nickel products. Additionally, the metallurgical process used for nickel extraction from one type of laterite nickel ore cannot be directly applied to another type (de Alvarenga Oliveira V et al., 2020). The metallurgical processes for laterite nickel ore can be generally divided into four categories: Pyrometallurgy, hydrometallurgy, pyro-hydro combined methods, and other processes, with the first two processes being the most commonly used in commercial production.
Limonite ores have high iron content, low nickel, magnesium and silicon content, high cobalt content, poor crystallinity, and a relatively loose structure, which is more suitable for hydrometallurgical processes. Hydrometallurgical processes commonly include high-pressure acid leaching (HPAL), atmospheric leaching (AL), and heap leaching (HL) (Wu BQ et al., 2019; Zhao D et al., 2023). These processes have advantages such as low energy consumption and high comprehensive recovery rates, but also face challenges like high capital investment, low leaching rates, strict equipment and operational requirements, and tailings disposal issues.
Saprolite ores have high nickel, magnesium, and silicon content, but low iron and cobalt content, and their chemical composition and mineral makeup are highly uneven, which is more suitable for pyrometallurgical processes. Pyrometallurgical processes commonly include rotary kiln-electric furnace (RKEF), sulfide smelting, and oxygen-enriched side-blown (Wu BQ et al., 2020; Wang S et al., 2021). Pyrometallurgical processes have advantages such as shorter process flows, simpler operation, and higher efficiency, but they also have drawbacks, including high energy consumption and poor comprehensive metal recovery rates (Fig. 4; Table 3).
| Type | Process | Examples | Feed type | Lixiviant | T(°C)/ P(atm) |
Time | Advantages | Disadvantages | Product | Extraction/% | Recovery/% | Embodied energy (GJ/t Ni) |
GHG emissions (t CO 2 eq/t Ni) |
References |
| Hydrometallurgical process | High Pressure Acid Leaching (HPAL) | Ramu, Goro, Ambatovy | Limonite, w(Mg)<5%w(Ni)> 1.3%) |
H2SO4 HNO3 H3PO4 |
250‒270; 30‒50 atm | 90 min | Low energy consumption, low GHG emissions, high recovery | High equipment requirements, harsh operation, and high capital expenditure | Intermediate | 90‒95 | Ni: 95; Co: 90 | 272 | 19 | IEA, 2021; Pandey N et al., 2023; Stanković S et al., 2020 |
| Atmospheric leaching (AL) | Eaglebridge’s Kristiansund refinery in Norway | Limonite | H2SO4 HCl HNO3 |
85‒110; 1atm | 12 h | Low energy consumption, easy to operate and control | Low extraction, long leaching time and high acid consumption | Intermediate | 85‒95 | Ni: 98.2; Co: 98.6 | 167 | 14.6 | Pandey N et al. 2023; Stanković S et al., 2020; Tian QH et al., 2023 | |
| Heap Leaching (HL) | Piaui (Brazil) NiWest (Australia) Acoje (Philippines) Pearl (Indonesia) | Limonite-Saprolite | H2SO4 | Ambiental | 120‒ 150 d |
Low capital and operating costs, low energy consumption, easy operating | low recovery, higher acid consumption | Intermediate | 70‒80 | Ni: 86 | 211 | 17.6 | Pandey N et al., 2023; Stanković S et al., 2020 | |
| Direct Nickel (Dni) | Dni built a pilot test line in Perth, Australia; Altilium of UK plans to build a smelter in Indonesia | Saprolite | HNO3 | 105°C; 1atm | 2‒4 h | Simple plant maintenance, flexibility in feed, recycled nitric acid |
Strict acidity control, removal of dissolved Al during purification |
Intermediate | >90 | Ni: 100 | 380‒400 | 34‒36 | Pandey N et al., 2023; Stanković S et al., 2020; Khoo JZ et al., 2017; Zhao D et al., 2023 | |
| Pyrometallurgical Process | Rotary kiln - electric furnace (RKEF) | Sorowako/Pomalaa (Indonesia), Tagaung Taung (Myanmar), Dingxin (Tingshan) Cerro Matoso (Colombia) | Saprolite w(Ni) about 2%, w(Fe)< 20%) |
/ | 850–1000°C in Rotary Kiln 1500‒ 1600°C in Electric Furnace |
/ | High nickel recovery, short process and high efficiency | High energy consumption, large amount of slag, poor ore adaptability, high operating cost | ferronickel | / | Ni>90; Fe>90 | 592, 35000‒ 40000 kWh/t of nickel |
45 | Wei WJ et al., 2020; Nickel Institut, 2023; Tian QH et al., 2023; Shi ZY et al., 2019; Wang S et al., 2021 |
| Oxygen-enriched side-blown (OESB) | Morowali (Indonesia) | Limonite & Saprolite, | / | 1500°C; 1atm | / | Low cost, short construction period, flexible ferronickel grade, flexibility in feed, environmentally friendly |
The technology is difficult and the production efficiency remains to be seen | Nickel matte | / | Ni: 94.12; Fe: 52 | 10000 kWh/t of nickel | 33.75 | Chen XG et al., 2018 | |
| Sulfide smelting | Donniambo (New Caledonia) Soroako (Indonesia) |
Saprolite, strict requirements on magnesium content | / | 1500‒ 1600 |
/ | Mature technology, low equipment cost, easy operation, flexible product form | High energy consumption, high GHG, low recovery, poor feed adaptability | Nickel matte | / | Low nickel matte Ni: 8‒15 High nickel sulfur Ni: 70 | 59 | IEA, 2021; Wang S et al., 2021; Wu BQ et al., 2020; Li JH et al., 2015 | ||
| Pyro-hydrometallurgical combined process | Reduction roasting-ammonia leaching (Caron) | Yabulu (Austrila), Surigao&Berong (Philippines), Sukhinda (India), Yuanshishan (China) | Limonite & Saprolite, w(Mg)>10% | NH3-(NH4)2CO3 | 600‒900 °C; 1atm |
/ | Recyclable leaching agent, low cost | Low recovery, high energy consumption, poor operating environment | Nickel Oxide, Nickel Sulfate | Ni: 75‒80; Co<50 | Ni:91.2; Co:67.2 | 565 | 44.8 | Tian QH et al., 2023; Zhao D et al., 2023; Pandey N et al., 2023; Stanković S et al., 2020 |
| Selective reduction-hydrometallurgical magnetic separation (Krupp-Renn) | Oyama (Closed); Beihai Chengde Nickel Company | Saprolite w(Ni)>1.8% |
/ | 1400°C | / | Low energy consumption and low operating cost | Rotary kiln ringing, difficult operation, limited production | Nickel pig iron | / | Ni: 20‒25; Fe: 75-80 | Coal consumption per ton of ore: 170 tons 5000 kWh/ton of nickel | Tian QH et al., 2023; Wu BQ et al., 2020 | ||
| Other processes | Ferredox reductive bioleaching | Piaui (Brazil) (concept) | Limonite | H2SO4+acidophilic bacteria | 30°C; 1atm | 7d | High cobalt recovery, lower capital and operational expenses, lower CO2 emissions |
Lower process efficiency, lower nickel extraction |
/ | Ni: 66; Co: 95; Fe: 10; Mg: 50; Mn: 81 | Stanković S et al. 2020, 2024; Li JH et al., 2015 | |||
| Chloride Metallurgy | Limonite & Saprolite, | HCl | 100°C; 1atm | 30 min/ 90 min |
High nickel and cobalt extraction, relatively mild leaching conditions, acid and alkali renewable | Higher iron extraction, high acid consumption, highly corrosive hydrochloric acid requires a tougher equipment. | / | Limonite Ni: 99.6; Co: 100; Fe: 96.9; Saprolite Ni: 84.9; Co: 100 | Zhao D et al., 2023; Wang YY et al., 2023; Li JH et al., 2015; Stanković S et al., 2020 |
Pyro-hydrometallurgical combined processes combine the advantages of both methods, including reduction roasting-ammonia leaching (Caron process), sulfuric acid roasting-water leaching, and selective reduction - magnetic separation (Shi ZY et al., 2019). Other processes include bio-hydrometallurgy, chlorometallurgy, and microwave pretreatment (Wang CY and Ma BZ, 2020).
The development and application of smelting processes are related to changes in the supply and demand structure of nickel, which can be roughly divided into three stages. The first stage was before 2007, when changes in nickel demand were mainly driven by stainless steel. From 1996 to 2007, the compound annual growth rate (CAGR) of nickel demand for stainless steel was 3.05%. During this phase, nickel mainly came from electrolytic nickel produced through the pyrometallurgical smelting of sulfide nickel ores. The second stage was from 2007 to 2017, during which the growth in nickel demand was primarily due to China’s significant increase in stainless steel consumption, with a CAGR of 5.7% in nickel demand for stainless steel during this period. At this time, the supply of sulfide nickel ores was insufficient, and the pyrometallurgical smelting of laterite nickel ores developed rapidly, with a sharp increase in patent applications. During this phase, nickel mainly came from ferronickel and nickel pig iron produced through the pyrometallurgical smelting of laterite nickel ores. Around 2015, the demand for nickel sulfate driven by power batteries gradually increased, pushing patent applications for hydrometallurgical smelting processes for laterite nickel ores to their peak (Yu HJ et al., 2022). The third stage, from 2018 to the present, has seen incremental nickel demand mainly come from the power battery sector. Intermediate products from the hydrometallurgical smelting of laterite nickel ores have become a significant source of nickel during this phase. The depletion of nickel resources and shifting demand patterns make research on nickel extraction from low-grade laterites crucial.
The hydrometallurgical process is primarily used for processing low-grade limonite ores and can be further divided into high-pressure acid leaching, atmospheric acid leaching, combined high-pressure and atmospheric acid leaching, heap leaching, etc. (Li JH et al. 2015; Pandey N et al. 2023). In recent years, there has been considerable research into hydrometallurgical processes, mainly focused on increasing the extraction rates of nickel and cobalt while reducing acid consumption, leading to the development of combined high-pressure and atmospheric acid leaching processes. Additionally, based on ore characteristics, renewable leaching media are being used to reduce acid consumption while recovering iron, such as in the nitric acid pressure leaching process, which enhances the comprehensive utilization of resources (Wang CY and Ma BZ, 2020). Among these processes, the high-pressure acid leaching (HPAL) process is the most mature and is the preferred hydrometallurgical method, having been implemented in projects such as MCC’s Ramu and Vale's Goro. Heap leaching is a simple process and has also been widely applied. However, combined high-pressure and atmospheric acid leaching, nitric acid pressure leaching, and the direct nickel process are still in the research and experimental stages.
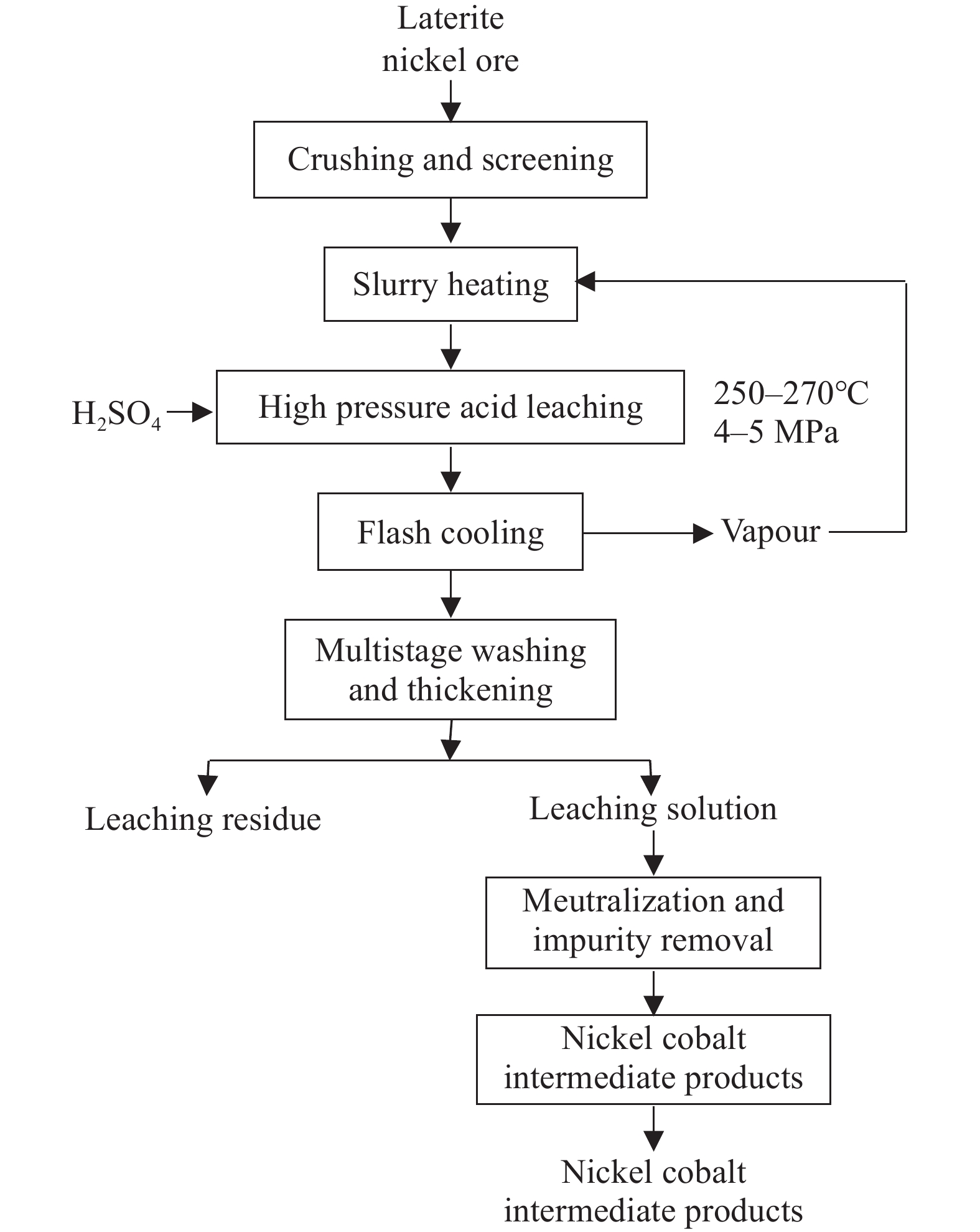
In the 1950s, HPAL process was firstly put into commercial production of nickel laterites at Moa Bay, Cuba. Since the 1990s, with the widespread use of autoclaves in gold refining, the HPAL process for nickel extraction has been further promoted (Fig. 5). Currently, projects using the HPAL process are listed in Table 4. As shown in Table 3, HPAL process requires a 30‒50 atm pressure and a temperature of 250°C‒270°C. Sulfuric acid solution is commonly used as a lixiviant to leach Ni, Co, Fe, Al and other metal elements from laterite nickel ore, as described in reactions (1)–(5) (Pandey N et al. 2023; Stanković S et al., 2020). Afterward, impurities such as Fe, Al, and Si undergo hydrolysis reactions (6)–(8) by controlling the pH value within the range of 2.0–2.5, forming precipitates that enter the residue, while Ni and Co selectively enter the leachate. The leachate is further separated through sulfide precipitation or neutralization precipitation, resulting in a nickel-cobalt intermediate product with high Ni and Co content, which can either be sold directly or further processed using traditional refining methods to produce electrolytic nickel or nickel sulfate (Zhao D et al., 2023; Qu T et al., 2020; Fig. 5).
| Company Name | Plants or projects/country | Process and products | Nickel-cobalt production scale/kt |
Investment amount (B$) and start-up time |
| Sherritt | Cuba/Moa | HPAL/MSP | 32; 2 | -; 1959 |
| Glencore | Murrin Murrin/Australia | HPAL/MSP | 36.6; 3 | 1; 1998 |
| Sumitomo Metal | Rio Tuba (Coral)/Philippines | HPAL/MSP | 22; 1.5 | 0.487; 2005 |
| First Quantum | Ravensthorpe/Australia | HPAL/MHP | 25; 14 | 2.2; 2008 |
| Prony Resources | Goro/New Caledonia | Nickel Oxide & Cobalt Carbonate | 60; 5 | 5.8; 2009 |
| MCC | Ramu/Papua New Guinea | HPAL/MHP | 32; 3 | 2.04; 2012 |
| Sumitomo Metal/Sherritt | Ambatovy/Madagascar | Nickel & Cobalt Briquettes | 60; 5 | 5.5; 2012 |
| Sumitomo Metal | Taganito/Philippines | HPAL/MSP | 36; 2.6 | 1.7; 2013 |
| Liqin/Harita | Obi Island/Indonesia | HPAL/MHP | 37; 4.5 | 0.7; 2021 |
| Huayou / Tsingshan | Morowali/Indonesia | HPAL/MHP | 60; 7.8 | 1.28; 2022 |
| Tsingshan/Gelinmei/Bangpu | Morowali/Indonesia | HPAL/MHP | 50; 4 | 0.998; 2022 |
| Huayou/Tsingshan/Yiweilinng | Weda Bay/Indonesia | HPAL/MHP+MSP | 120; 15 | 2.08; 2023 |
| Eramet/BASF | Weda Bay/Indonesia | HPAL/MHP | 42; 5 | -; 2025 |
| Vale/Sumitomo Metal | Pomalaa/Indonesia | HPAL/MSP | 40; - | -; 2026 |
|
NiO+H2SO4=NiSO4+H2O |
(1) |
|
CoO+H2SO4=CoSO4+H2O |
(2) |
|
2FeO⋅OH+H2SO4=Fe2(SO4)3+4H2O |
(3) |
|
Fe2O3+3H2SO4=Fe2(SO4)3+3H2O |
(4) |
|
Al2O3+3H2SO4=Al2(SO4)3+3H2O |
(5) |
|
Fe2(SO4)3+H2O=Fe2O3↓+3H2SO4 |
(6) |
|
Al2(SO4)3+6H2O=2Al2O3↓+3H2SO4 |
(7) |
|
Ni2SiO4+2H2SO4=2NiSO4+SiO2↓+2H2O |
(8) |
|
MgCO3+H2SO4=MgSO4+H2O+CO2↑ |
(9) |
|
MgO⋅SiO2+H2SO4=MgSO4+H2SiO3 |
(10) |
The advantage of this process is that during the hydrolysis of iron and aluminum ions, the hydrogen ions consumed during dissolution are released back into the liquid phase, ensuring efficient leaching of nickel and cobalt while reducing acid consumption. The nickel and cobalt recovery rates can reach 90%–95%, but the process has strict requirements for the composition and mineralogy of laterite nickel ore. It is suitable for processing limonite with MgO < 5%, Ni > 1.3%, and low aluminum content (Table 3; Wang CY and Ma BZ, 2020). It is generally believed that serpentine-type ores with high magnesium and calcium content are not suitable for this process due to increased acid consumption, which raises costs, as shown in reactions (9)–(10). Reducing acid consumption has thus become a direction for improving the HPAL process. However, the research by Miao Z et al. (2020) shows that an increase in magnesium content in the raw ore can reduce costs. This is because ores with higher magnesium content also tend to have higher nickel content, which reduces overall costs. The combined high-pressure and atmospheric acid leaching process follows this idea to improve acid utilization . Whether it is the early high-pressure-atmospheric combination or the later atmospheric-high-pressure combination, or the enhanced pressure acid leach (EPAL) process improved by BHP, all use sulfuric acid to leach low-magnesium limonite ores first, then use the free acid in the leachate to leach high-magnesium serpentine ores, thus improving acid utilization (Tian QH et al., 2021).
In addition, Ma Baozhong's team developed a nitric acid pressure leaching process for the Yuanjiang limonite ores of Yunnan Province, and has completed pilot-scale testing (Ma BZ et al., 2011). The leaching conditions for this process are relatively mild, with nickel and cobalt extration ≥ 95%, recovery > 92%, iron is produced as iron concentrate with a grade of over 60%, the nitric acid recovery rate more than 97%, and the NOx concentration in the exhaust gas after absorption is less than 50 mg/m³ (Wang CY et al., 2019).
HPAL process has undergone three technological iterations: the Moa model with vertical non-mechanical agitation autoclaves, the Murrin Murrin model with horizontal multi-compartment mechanically agitated autoclaves, and the Ramu model with three-stage preheating and three-stage flash evaporation. It has now become the mainstream hydrometallurgical process for nickel extraction from laterite nickel ores. This process has significant advantages in processing limonite with high cobalt and iron content, offering low energy consumption and exceeding 90% nickel and cobalt recovery rates.
The HPAL process is technically challenging, mainly in three aspects: First, the physical properties of the ore greatly affect the stability and efficiency of the smelting process, requiring project designs tailored to the nature of the raw ore, making it difficult to replicate systematically (Miao Z et al., 2020); second, due to the high-pressure and high-temperature leaching environment, core equipment must be made of special materials, such as titanium alloys containing palladium for the autoclaves; third, extracting one ton of nickel metal generates 150–200 t of acidic tailings, necessitating proper tailings management. Additionally, the HPAL process has high production losses, long construction periods, and high plant construction costs. Issues such as corrosion and scaling frequently occur during production, requiring regular maintenance for the equipment. Duman BO and Can IB (2022) shows that adding acid to the autoclave in three stages can effectively reduce scaling.
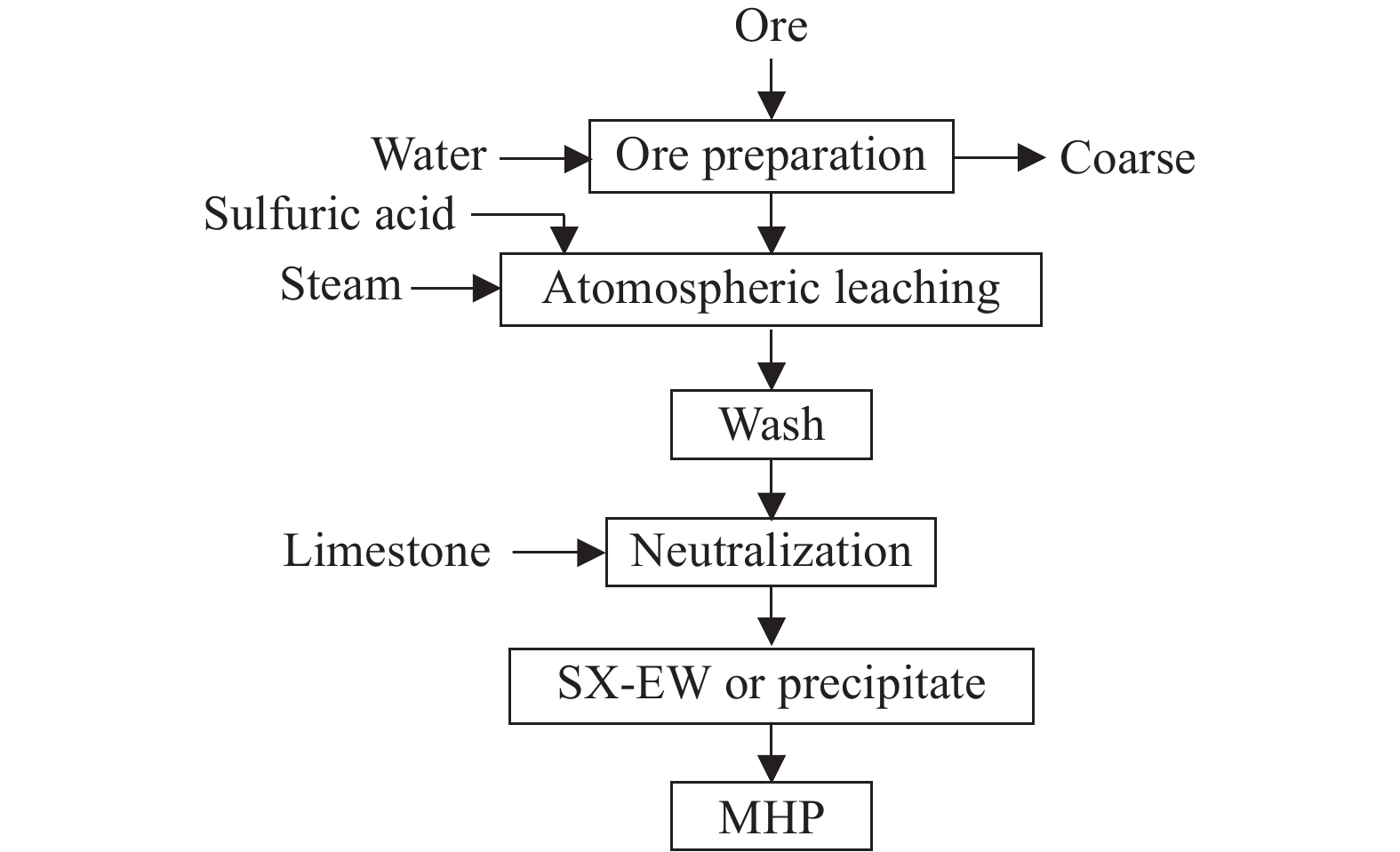
Atmospheric acid leaching is the process of extracting target metal elements or compounds into an acidic solution under normal temperature and pressure conditions. Commonly used acidic solutions include sulfuric acid, hydrochloric acid, and nitric acid. The main process of atmospheric sulfuric acid leaching for laterite nickel ore is shown in Fig. 6. It can be specifically divided into the following steps: (1) Crushing, grinding, screening, and pulping; (2) Mixing the slurry, washing solution, and sulfuric acid in a certain proportion, controlling the leaching temperature to leach out elements such as nickel and cobalt from the ore; (3) Neutralizing with lime powder or other neutralizing agents and performing solid-liquid separation; (4) Treating the solution with sodium sulfide or hydrogen sulfide to separate and obtain nickel-cobalt sulfides (Pandey N et al. 2023) .
Compared with HPAL, AL has gained attention due to its milder leaching conditions. However, the extraction of nickel is lower than that of HPAL, and thus surfactants are used to improve nickel leaching efficiency. Zhang PY et al. (2019) significantly improved the extraction of nickel and cobalt by using stearyltrimethylammonium chloride (STAC) at 100°C, with an ore-to-surfactant ratio of 15∶1 and a leaching time of 8 hours. Additionally, sodium sulfite can also accelerate the extraction of iron from goethite and the release of nickel in atmospheric sulfuric acid leaching (Luo J et al. 2015).
Guo H et al. (2020) achieved nickel, cobalt, and iron extraction of 96.27%, 92.2%, and 81.57%, respectively, under the conditions of a sulfuric acid concentration of 2 mol/L, a leaching time of 120 minutes, a leaching temperatureof 80°C, and a liquid-to-solid volume ratio of 11 mL/g. The leaching process was controlled by a combination of chemical reactions and diffusion, with an activation energy of 25.867 kJ/mol.
Guo Q et al. (2015) proposed an innovative technique involving atmospheric hydrochloric acid leaching and spray hydrolysis for processing saprolite ore from the Philippines. Under optimal leaching conditions, the extraction of Ni, Fe, and Mg were 98.9%, 97.8%, and 80.9%, respectively. When processing laterite nickel ore using atmospheric hydrochloric acid leaching, the mineral lattice structure is destroyed, and the main product of the leaching residue is silicon dioxide. The nickel grade in the hydrolysis solution is 4.55%, which can be used for the production of ferronickel.
Li JH et al. (2019) used an ammonium chloride-hydrochloric acid system to selectively leach low grade saprolite ores. Under the conditions of a leaching temperature of 90°C, a hydrochloric acid concentration of 2 mol/L, a solid-to-liquid ratio of 1∶6, and a leaching time of 90 minutes, the extraction of Ni, Co, and Mg were 89.45%, 88.56%, and 90.23%, respectively, while the extraction of iron was 19.30%. The addition of chlorides promoted the dissolution of goethite, but had little effect on other iron phases.
The Australian Direct Nickel Group developed an atmospheric pressure nitric acid processing for laterite nickel ore, known as the DNi process. This process can be used for all kinds of laterite nickel ore. The key to the process is replacing sulfuric acid with pure nitric acid, utilizing patented technology that recycles over 95% of the nitric acid, thus lower operation costs and more friendly environment. The main products produced by this process are mixed hydroxide precipitate (MHP) or mixed nickel-cobalt oxide (MOP), as well as by-products such as Fe and MgO (Xu CL et al., 2021; Zhao D et al., 2023). Currently, a feasibility study is being conducted for building a plant at Tanjung Buli of Halmahera Island, Indonesia. A final feasibility study is also underway at the Townsville Energy Chemicals Hub refinery in northern Queensland, Australia.
The advantages and disadvantages of different lixiviants in AL process are shown in Table 5. Compared with HPAL, AL has a lower energy consumption and is easy to operate and control, but its disadvantages such as low extraction, long leaching time and difficult separation of leachate, etc. still need to improve (Table 3).
| Acid solution | Advantages | Disadvantages |
| Sulfuric acid | Direct heap leaching, operate easily | Large acid consumption acid can not be recycled, low leaching efficiency |
| Nitric acid | High Ni leaching rate, nitric acid can be recycled | Prone to produce toxic gas NOx |
| Hydrochloric acid | The separation of Ni, Co is easy, hydrochloric acid can be recycled | High corrosiveness, high requirements of equipment |
| Organic acid & microbial derived acid | Low leaching temperature, operate easily and environmental friendliness | Long operating time, low efficiency |
The heap leaching process originated in the 1960s, primarily used to process low-grade copper ores that conventional beneficiation could not economically work. It has been widely adopted and promoted for processing laterite nickel ores for its lower capital and operation costs.
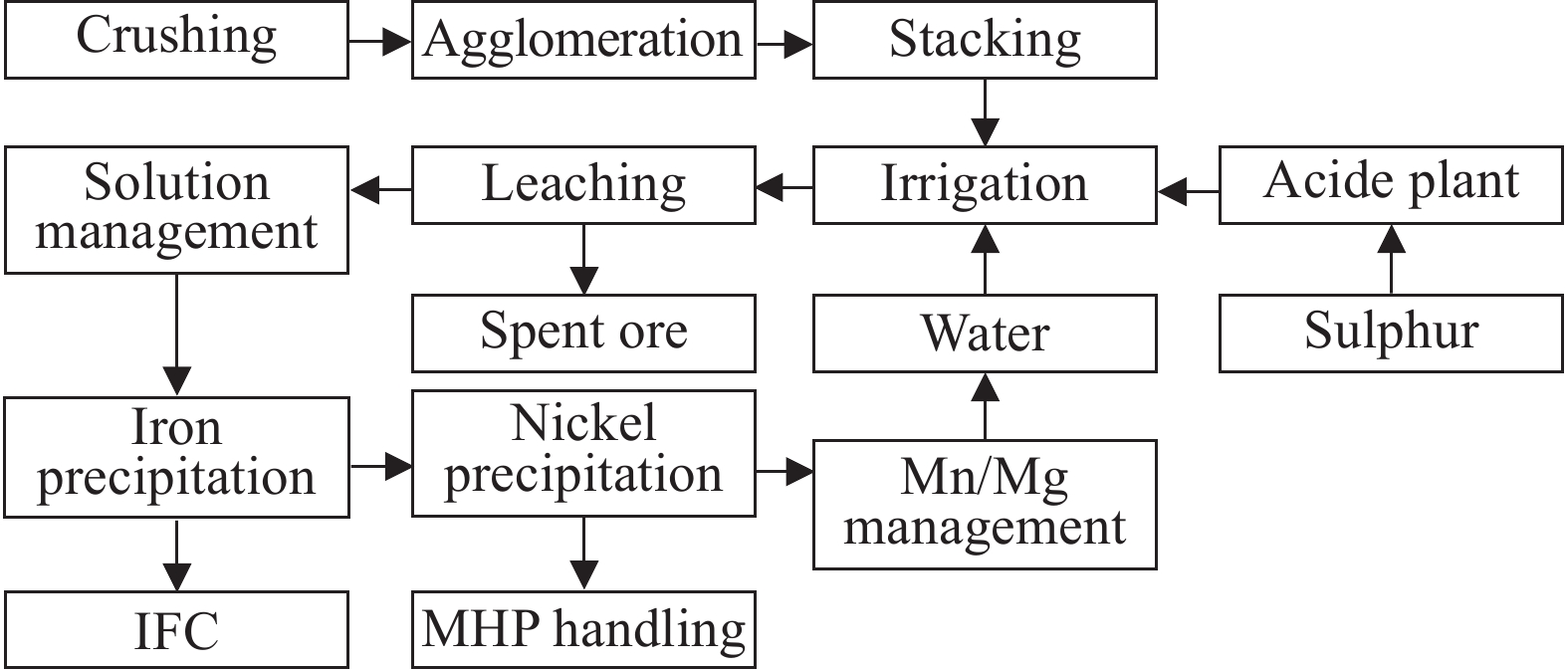
The feasibility of heap leaching mainly depends on the permeability of the ore and the leachability of valuable metals and impurities. HL process is suitable for processing saprolite ores with MgO content greater than 15% and relatively simple occurrence states of nickel and cobalt. The process involves crushing the saprolite into particles of 0.15 mm to 2 mm and directly placing them into the heap. spraying or dripping lixiviant to the heap, collecting the leachate, removing impurity and extracting nickel and cobalt to produce nickel and cobalt intermediates (Fig. 7; Oxley A et al. 2016).
Greece was the first country to investigate HL to treat nickel laterites, later confirmed to be applicable for saprolites from different regions (Stanković S et al., 2020). Two commercial application cases reported in the literature include Glencore's Murrin Murrin project in Australia, which has processed 1.5×106 t ore combined with HPAL, and the Yuanjiang project in Yunnan, China, which officially began production in 2005. The Yuanjiang nickel laterites has an MgO content of up to 28%, and the nickel occurrence is extremely complex. To reduce investment costs, HL process with an annual production capacity of 1000 t electrolytic nickel was developed and constructed, reaching a total production of 10000 t nickel metal by 2014 (Pandey N et al. 2023). European Nickel leached over 5000 t nickel laterites at the Acoje project in the Philippines. BHP leached over 20000 t nickel laterites at Cerro Matoso, in the meanwhile Vale, Anglo American, and Strata conducted various heap leaching tests (Table 6; Oxley A et al., 2016).
| Project | Country | Owner | Estimated cost USM$ | Planned Ni production capacity(ktpy) | Capital intensity US$/lb Ni |
| Piaui | Brazil | Brazilian Nickel | 450 | 22 | 9.28 |
| NiWest | Australia | GME | 400 | 14 | 12.75 |
| Cerro Matoso | Colombia | BHPB | 750 | 20 | 17.01 |
| Caldag | Turkey | ENK | 450 | 20 | 10.30 |
| – | Guatemala | BHPB | 2550 | 79.5 | 15.44 |
| Pearl | Indonesia | BHPB | 800 | 32 | 11.11 |
| Gag Island | Indonesia | BHPB | 800 | 27.3 | 13.47 |
| Cleopatra | USA | RFN | 475 | 21.5 | 10.02 |
| Acoje | Philippines | ENK | 498 | 24.5 | 9.22 |
Generally, the iron content in the leachate from HL is much higher than that from HPAL. Although HL has advantages such as simplicity and low investment, it has several drawbacks: (1) Low extractions for nickel and cobalt; (2) poor metal leaching selectivity, making purification processes complex; (3) high sulfuric acid consumption per ton of nickel (50–70 t); (4) difficult-to-treat leachate, causing significant environmental impact; and (5) slow leaching rate and lower recoveries of Ni and Co, making large-scale production challenging. These factors limit the application and scale of HL processes.
The saprolite ores with higher Si, Mg and lower Fe content are suitable for pyrometallurgical processes (Table 1). Traditional pyrometallurgical processes mainly include the ferronickel process and the nickel matte process, with their basic flow outlined in Fig. 8 (Li JH et al., 2015). The ferronickel process includes rotary kiln-electric furnace (RKEF) and blast furnace smelting, etc.; the nickel matte process includes blast furnace sulfide smelting, rotary kiln sulfide smelting, ferronickel converter sulfide smelting, and oxygen-enriched side-blown furnace sulfide smelting (Tian QH et al., 2023). This paper will focus on the mainstream pyrometallurgical processes in recent years.
The smelting temperature of nickel laterite is related to the SiO₂/MgO and the contents of FeO, Al₂O₃. When 1.8 < SiO₂/MgO < 2.2, the smelting temperature is lower (< 1600°C), making it suitable for producing nickel matte. A SiO₂/MgO ratio either <2 or >2.5 results in a higher smelting temperature, suitable for producing ferronickel. Ores in the SiO2/MgO ratio in the intermediate range (2.3‒2.5) are very corrosive to the furnace lining and require alteration to feed chemistry (by blending or fluxing) before they can be smelted (Dalvi AD et al., 2004). However, Palovaara P and Pisilä S et al. (2021) pointed out that due to factors like yield and cost, fluxing agents have been abandoned, providing parameter settings for pyrometallurgical smelting with SiO₂/MgO ratio 2.2 – 2.7. For limonites with nickel grades > 2.2%, Fe/Ni ratio of 5 to 6, high Mg content, it is suitable for producing high-carbon ferronickel, as seen in Doniambo, the Japan Nickel Smelting Plant, and Pomalaa in Indonesia. For saprolites with nickel grades > 1.5%, Fe/Ni ratios of 6 to 12, high Mg (Falcondo, Dominican Republic) or high Si (Cerro Matoso, Colombia), it is suitable for producing low-carbon ferronickel. For laterites with Fe/Ni > 6 and 1.8 < SiO₂/MgO < 2.2, it is suitable for producing nickel matte, such as at P.T. Vale (Dalvi AD et al., 2004).
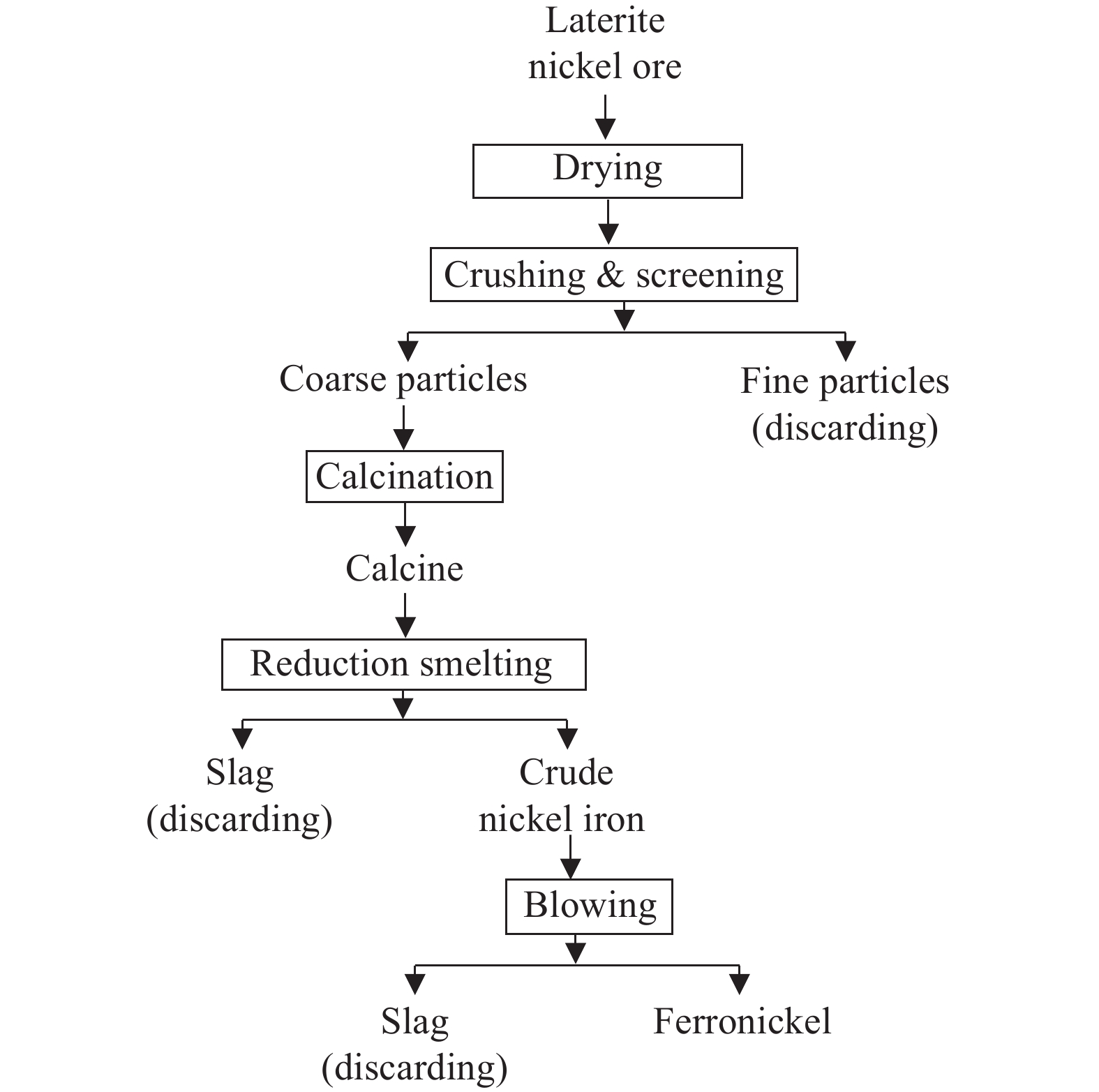
The RKEF process was originally developed at the Doniambo plant in New Caledonia in the 1950s and is currently the most widely used pyrometallurgical process for nickel laterites (Table 7). The RKEF process is mainly used to process saprolites with nickel content greater than 1.8%, Fe/Ni, Ni/Co and SiO2/MgO ratios of <12, >30 and 1.6–1.9, respectively (Wang CY and Ma BZ, 2020; Oxley A et al., 2016), and produces nickel pig iron containing 8–12% nickel, with a nickel recovery rate of more than 90%. The RKEF process is as Fig. 9. The laterite nickel ore is crushed and screened to a particle size of 50‒150 mm, and feed into a drying kiln to remove free water; then it is roasted and pre-reduced at a temperature of 850°C‒1000°C in a rotary kiln to remove crystal water; the high-temperature charge is directly feed into an electric furnace for reduction smelting at a temperature of 1500°C‒1600°C to produce crude nickel iron with >8% nickel, and then further refined in a converter or electric furnace to yield ferronickel with >25% nickel, used in stainless steel production (Wu BQ et al., 2020; Tian QH et al., 2023).
| Property | Country | Owner Name | Start Up | Capacity/kt Ni | Production Forms | Processing |
| Codemin | Brazil | Anglo American plc | 1982 | 10 | Fe-Ni | Smelting |
| Barro Alto | Brazil | Anglo American plc | 2011 | 41 | Fe-Ni | RKEF |
| Onca Puma | Brazil | Vale SA | 2011 | 27 | Fe-Ni | RKEF |
| Cerro Matoso | Colombia | South32 Limited | 1982 | 50 | Fe-Ni | Smelting |
| Falcondo | Dominican | Global Special Opportunities Ltd. | 1971 | 29 | Fe-Ni | Smelting |
| Pomalaa | Indonesia | PT Aneka Tambang Tbk | 1975 | 27 | Fe-Ni | Smelting |
| Weda Bay/Youshan | Indonesia | Chengtun Mining Group Co., Ltd. | 2020 | 34 | Fe-Ni | RKEF, Blast Furnace |
| SLN/Doniambo | New Caledonia | ERAMET SA | 1879 | 55 | Matte; Fe-Ni | Smelting |
| Koniambo | New Caledonia | Societe Miniere du Sud Pacifique SA | 2013 | 55 | Alloy | Smelting |
| Larco/Larymna plant | Greece | Larco SA | 1994 | 25 | Fe-Ni | RKEF |
| Tagaung Taung Smelter | Myanmar | China Nonferrous Metal Mining (Group) Co., Ltd. | 2011 | 22 | Fe-Ni | RKEF |
| Hachinohe Smelter | Japan | Pacific Metals Co., Ltd. | 1966 | 40.8 | Fe-Ni | RKEF |
| Hyuga Smelter | Japan | Sumitomo Metal Mining Co., Ltd. | 1956 | 22 | Fe-Ni | Smelting |
| Loma de Niquel Smelter | Venezuela | Federal Corporacion De Minas | 2000 | 17 | Fe-Ni | Smelting |
| Pobuzhskiy Plant | Ukraine | Solway Investment Group Limited | 2003 | 22 | Fe-Ni | Smelting |
| Sorowako | Indonesia | PT Vale Indonesia Tbk | 1977 | 80 | Matte | Smelting |
| Morowali/ZhongTsing Smelter | Indonesia | CNGR Advanced Material Co.,Ltd. | 2023 | 30 | Matte | Oxygen-enriched side blowing |
| Oheyama Smelter | Japan | Nippon Yakin Kogyo Co., Ltd. | 1939 | 10 | Fe-Ni | Krupp-Renn |
| Piaui | Brazil | Brazilian Nickel Plc | 2016 | 1 | Hydroxide | Acid Leach |
| Punta Gorda | Cuba | Cubanique | 1986 | 30 | Ni Oxide | Caron |
| Nicaro Smelter | Cuba | General Nickel Co SA | 1981 | 15 | NiO | Caron |
| Yabulu Refinery | Australia | Queensland Nickel Pty Ltd. | 1974 | 32 | Hydroxide | Caron |
The reaction of the rotary kiln pre-reduction process is as follows (Dong B et al., 2023):
|
2C+O2=2CO |
(11) |
|
NiO+C=Ni+CO |
(12) |
|
Ni+CO=Ni+CO2 |
(13) |
|
3Fe2O3+CO=2Fe3O4+CO2 |
(14) |
|
Fe3O4+CO=FeO+CO2 |
(15) |
The reaction of the electric furnace reduction smelting process is as follows:
|
2C+O2=2CO |
(16) |
|
FeO+CO=Fe+CO2 |
(17) |
|
SiO2+C=Si+CO2 |
(18) |
The main issue with the RKEF process is the high operation costs resulting from carbon emissions and high energy consumption. The high contents of Al2O3, MgO, and SiO2 in saprolites can lead to the formation of high-melting-point phases, which affect the properties of the melt, requiring a smelting temperature of 1550°C or higher (Dong B et al., 2023). This results in electricity costs accounting for about 50% of the operation costs (Qu T et al., 2020). To this end, Li GH et al. (2017) strengthened the reducing atmosphere during the pre-reduction and smelting process can lower the initial liquid phase formation temperature of the lateritic nickel ore in the electric furnace, thereby reducing the smelting temperature by 50°C–90°C, which effectively decreases the electricity consumption required for smelting ferronickel and operation costs by 5%–10%. Wang QM et al. (2020) and Yu DW et al. (2020) directly reduced and smelted ferronickel using hydrogen gas, subsequently using mono- or bi-metallic eutectic of zinc and magnesium to selectively extract nickel from the ferronickel at high temperatures. Finally, they employed vacuum distillation to separate nickel from the metallic eutectic based on differences in the saturation vapor pressures of different metals, producing nickel powder, which effectively address the issues of high carbon emissions and energy consumption in the current RKEF process. Additionally, there are practices of converting rotary kilns to bottom-blown furnaces (Qu T et al., 2020). While this can reduce energy consumption by 16.64% and costs by 10.88%, it also has drawbacks, such as small production capacity and high failure rates that need to be addressed (Wang S et al., 2023).
The RKEF process is mainly suitable for processing serpentine and saprolites to obtain high-nickel-content ferronickel, while research on improving the quality of limonite using this process is relatively limited. This is due to limonite with a high Fe/Ni ratio, which leads to non-selective reduction and ultimately produces low-grade ferronickel products.
Wang X et al. (2023) found that the metallization rate of iron during pre-reduction is a crucial factor affecting the nickel grade of ferronickel. A limonite ore with 0.98% Ni from the Philippines was used to selective reduction smelt to produce ferronickel. They recommended an iron metallization rate of 10.93%, a nickel metallization rate of 94.3%, an alkalinity of 0.60, and an MgO/SiO₂ ratio of 0.30, smelting at 1525°C for 45 minutes. The resulting ferronickel had Ni and Fe grades of 12.55% and 84.61%, with Ni and Fe recovery rates of 85.65% and 10.87%, respectively. The contents of S and P in the ferronickel were only 0.11% and 0.0035%, respectively. The selective reduction smelting process can significantly enhance the nickel grade of ferronickel products derived from limonite ore.
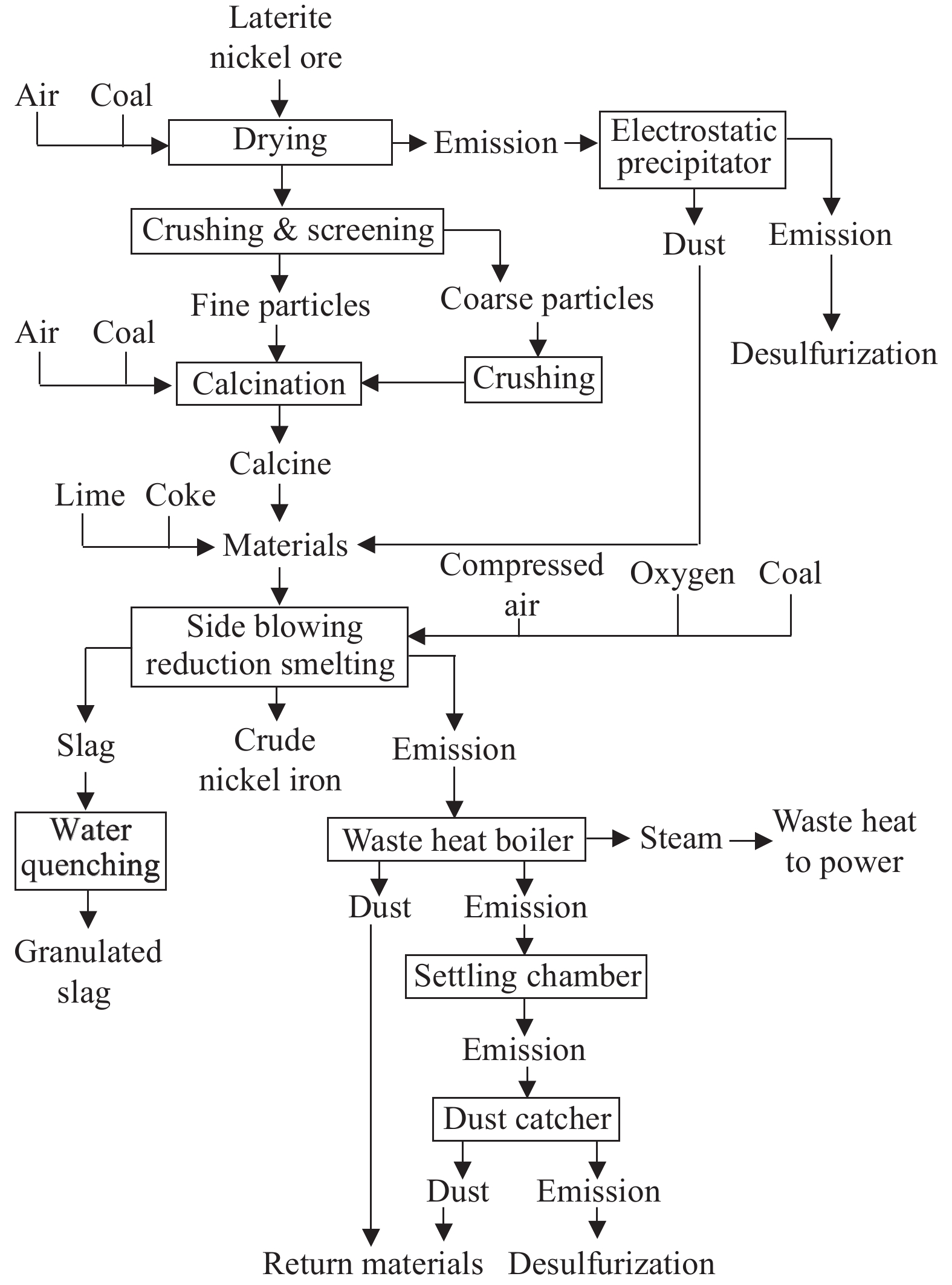
Oxygen-enriched side-blown smelting technology is an improvement based on the Vanyukov smelting process from Russia and is one of the modern smelting techniques for molten pools . The Vanyukov smelting process has been successfully applied in the smelting of non-ferrous metals such as copper and lead-zinc ores, and research on its application in lateritic nickel ore smelting has been conducted in the past decade (Keskinkilic E, 2019). The OESB is an enhanced smelting techniques for molten pools which uses multi-channel side-blown lances to inject oxygen-rich air and fuel (natural gas, producer gas, pulverized coal) into the molten pool at subsonic speeds. The vigorous stirring caused by the air blast quickly immerses the materials in the melt to finish the physical and chemical reactions (Chen XG et al., 2018). The main differences between OESB and RKEF are the changes of sulfide method, the substitution of Vanyukov smelting furnace for the electric furnace, the more feed selectivity which allows to process medium- and low-grade nickel laterites (Zhang ZF et al., 2022) .
The OESB process flow as show in Fig. 10. The ore is first dehydrated in a drying kiln using pulverized coal as fuel, then crushed and screened before undergoing deep drying and roasting in a rotary kiln. The dried lateritic nickel ore, reducing coal, flux, and recycled smelting dust are then fed into the Vanyukov smelting furnace. Subsequently, about 70% of the oxygen-enriched air and pulverized coal are introduced into the molten pool through submerged lances on its both sides. The submerged combustion flame directly contacts the melt, while the injected oxygen-rich air and coal powder stir the molten pool, enhancing heat transfer and accelerating the reaction, allowing the feeds to melt rapidly. Reducing coal is added from the top of the furnace to reduce high-nickel slag, resulting in the reduction of 90%–95% of nickel and 40%–50% of iron to form crude nickel iron alloy. The remaining impurities and gangue are converted into slag, with the nickel content in the slag controlled to be less than 0.1%. The crude nickel iron alloy is then further smelted to produce ferronickel (Chen XG et al., 2018) .
Since August 2004, the South Ural Nickel Plant in Russia had been using the Vanyukov smelting process to treat low-grade nickel oxide ore and the expected targets were achieved in 2006. The nickel recovery rate reached 88%, with nickel content of 12.4% in low nickel matte and 0.17% in slag (Tong XW and Li YG, 2011). Tsymbulov LB et al. (2011) conducted pilot experiments using the Vanyukov smelting process on limonite ore from the Kemprisay, in Kazakhstan, producing ferronickel with a nickel content of 20% and a nickel recovery rate exceeding 90%. This process is suitable for high Fe/Ni ores. Keskinkilic E (2019) performed pilot experiments on the saprolite ore from Buruktal, Russia.
The improved OESB smelting technology has been successfully applied in the smelting of non-ferrous metals such as copper and lead in China, and engineering practices have also been initiated for lateritic nickel ore, nickel sulfide ore, and nickel-containing waste (Tong XW and Li YG, 2011). Zhongwei Co., Ltd. has successfully launched its first OESB high nickel matte production line in the Morowali Industrial Park in Indonesia, with an annual capacity of 10000 t of nickel metal, which went into operation at the end of 2022. As the side-blown furnace continues to be optimized in terms of structural design, processing capacity, furnace life, operational mechanization, and control of process parameters, its advantages will become further apparent (Yuan JH, 2022) .
Chen XG et al. (2023) conducted the research on the selective reduction sulfidation of lateritic nickel ore using OESB smelting with gypsum as a sulfurizing agent. This method effectively utilizes the Ca and S components in gypsum, thereby improving sulfur utilization, reducing sulfur volatilization, and achieving comprehensive utilization of industrial solid waste residues. The experiments showed that the recovery rate of nickel and cobalt exceeded 92% and 88%, and the sulfur utilization rate surpassed 75%, while the iron recovery rate was below 58%. Under conditions controlling the C/S ratio and satisfying the carbon requirements for reduction, Gypsum enables selective reduction sulfidation of lateritic nickel ore to produce low-nickel matte.
The OESB sulfide smelting nickel matte process has advantages such as broad feed selectivity, flexible product control, low investment, low energy consumption, and environmental friendliness,
making it an important development direction for future pyrometallurgical processes of nickel laterites. However, the limitations of the OESB process are evident in the subsequent converter refining of high nickel matte, where significant cobalt losses occur, resulting in a cobalt recovery rate that is markedly lower than that of hydrometallurgical processes.
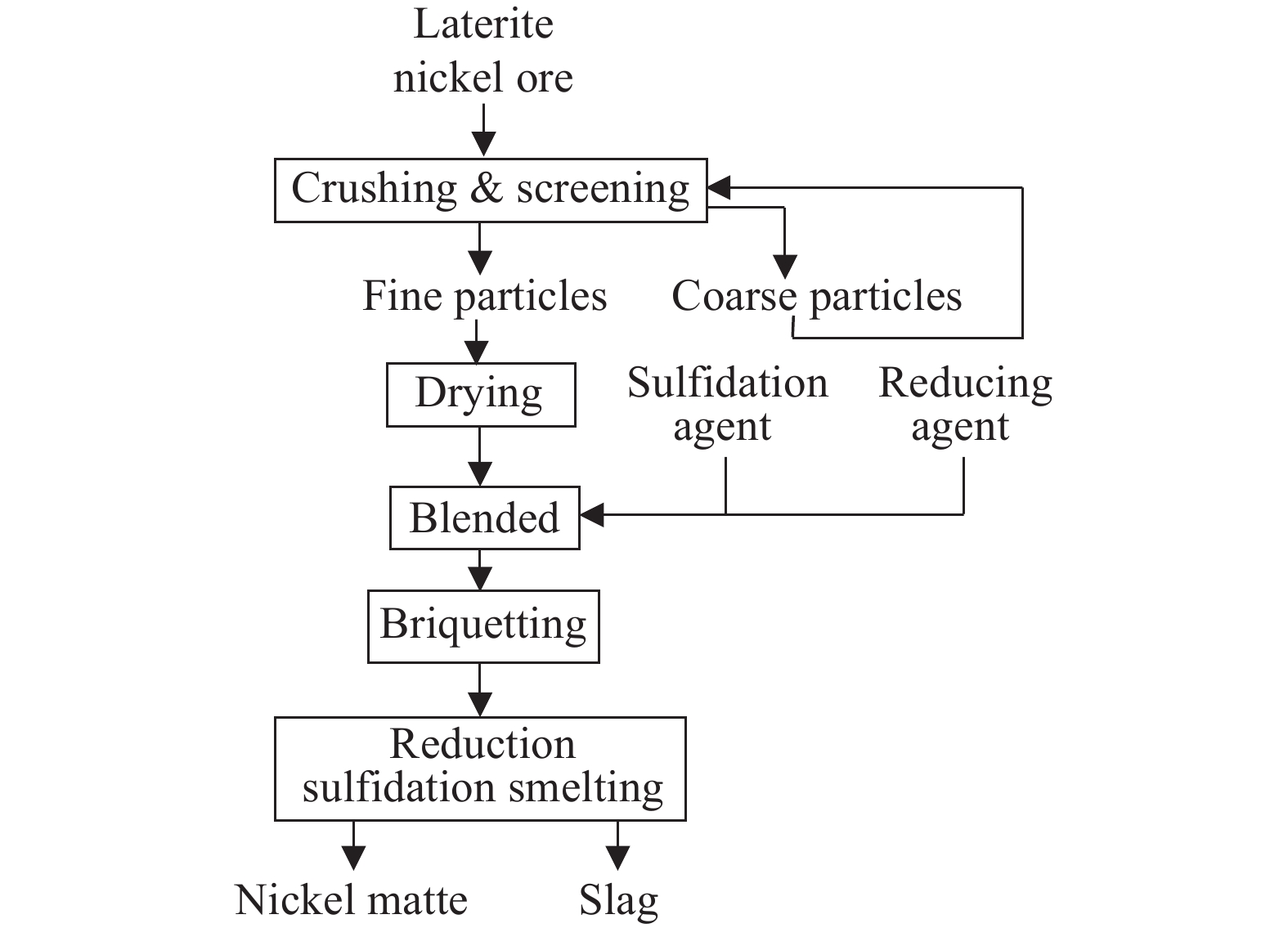
Blast furnace smelting for nickel matte is a mature process for treating nickel sulfide ores, which began commercial production of nickel laterites in the 1920s and 1930s, making it one of the earliest pyrometallurgical processes for nickel laterites. This process is suitable for low-nickel laterites with a SiO₂/MgO ratio of 1.8 to 2.2 (Dalvi AD et al., 2004). The main workflow of the sulfidation smelting nickel matte process involves crushing, drying, and screening the lateritic nickel ore to remove free water and some crystallization water. Sulfurizing agents (such as sulfur, pyrite, or gypsum) and reducing agents (such as powdered coke) are added for mixing and briquetting. The briquettes are then smelted in a blast furnace or electric furnace at temperatures of 1500°C to 1600°C, yielding low-nickel matte with a nickel content of 8%–15%. During the smelting process, the addition amount and ratio of sulfurizing and reducing agents are adjusted to control the grade of low-nickel matte, with a nickel recovery rate of approximately 85%. The low-nickel matte is then refined in a converter to produce high-nickel matte, which generally contains 70% nickel and 19.5% sulfur. The overall nickel recovery rate is about 70% (Fig. 11; Wang CY and Ma BZ, 2020).
The main reduction reactions in the sulfidation smelting process are (Dong B et al. 2023):
NiO+C=Ni+CO
NiO+CO=Ni+CO2
NiSiO3+2FeO+C=Ni+Fe2SiO4+CO
NiSiO3+2FeO+Co=Ni+Fe2SiO4+CO2
NiSiO3+2CaO+C=Ni+Ca2SiO4+Co
NiSiO3+2CaO+CO=Ni+Ca2SiO4+CO2
Fe3O4+CO=FeO+C2
2Fe3O4+Fe2SiO4+2CO=2FeO+2CO2+SiO2
FeO+CO=Fe+CO2
Fe2SiO4+2CO=2Fe+2CO2+SiO2
The sulfidation reactions are as follows:
CaSO4·2H2O=CaO+SO3+2H2O
3NiO+9CO+2SO3=Ni3S2+9CO2
2NiSiO3+9CO+2SO3=Ni3S2+9CO2+3SiO2
FeO+4CO+SO3=FeS+4CO2
Fe2SiO4+8CO+2SO3=2FeS+8CO2+SiO2
3NiO+2FeS+Fe=Ni3S2+3FeO
6NiSiO3+4FeS+2Fe=2Ni3S2+3Fe2SiO4+3SiO2
NiO+Fe=Ni+FeO
2NiSiO3+2Fe=Fe2SiO4+SiO2+2Ni
The above reactions indicate that this process generates a large amount of sulfur dioxide leading to severe environmental pollution. Although the equipment for this process is relatively simple, the technology is mature, and the products can be adjusted, its drawbacks, such as high energy consumption, low nickel recovery rates, and high operation costs due to extensive use of coke, remain challenging to overcome. Currently, the only plants using this process are the Doniambo smelter in New Caledonia and the Sorowako smelter in Sulawesi, Indonesia (Table 7).
Wang HY et al. (2023) proposed a method for producing nickel matte from saprolite ore using CaS as a sulfurizing agent at 1500°C. In an argon atmosphere, CaS is prepared by reducing CaSO₄ with graphite. As the amount of CaS increases, nickel recovery rates rise while nickel grades decline. When the amount of CaS is six times the stoichiometric requirement for NiO sulfidation, the nickel recovery rate reaches 93.63%, with a nickel grade of 8.84% and a CaS utilization rate of 92.10%. Compared to using S₂ as a sulfurizing agent, using CaS can reduce SO₂ emissions and improve nickel recovery rates.
In light of the shortcomings of mainstream hydrometallurgical processes, such as high investment costs, acid consumption, and difficult tailings treatment, as well as the energy consumption, greenhouse gas emissions, and environmental pollution associated with pyrometallurgical processes, a combined process has been developed. The main feature of this process is the use of various types of additives for oxidation or reduction roasting at relatively low temperatures, which alters the structure, phase composition, or oxidation states of nickel and iron-bearing minerals (such as goethite and serpentine), improving their solubility and thereby enhancing the extractions of valuable metals (Li JH et al., 2019). Oxidation roasting typically occurs at 200°C–600°C, which changes the mineral’s structure, like goethite and serpentine, etc. through dehydration and decomposition reactions, increases the porosity and specific surface area of the mineral particles, and strengthens the leaching of nickel and cobalt under lower temperature and acid consumption conditions. Reduction roasting is usually performed at a temperature lower than those of blast furnaces and RKEF, selectively reducing nickel laterites to preferentially convert nickel and cobalt oxides to metallic states, while high-valence iron oxides are reduced to magnetite. Valuable elements are then further recovered through hydrometallurgical (ammonia leaching, acid leaching, water leaching) or physical methods (magnetic separation). The combined process effectively reduces energy and acid consumption.
Selective reduction for nickel laterite ore is one of a pyrometallurgical process carried out to reduce iron and nickel oxide in lateritic nickel ore into ferronickel by limiting the metallization of iron with additives (Nurjaman F, 2020). Additives are used to increase the grain size of the ferronickel particle and reduce the activation energy for its growth, facilitating separation from the slag and thus improving nickel grade and recovery rate. Currently, the additives used for selective reduction mainly include sulfates (Na2SO4, CaSO4, Na2S2O3), carbonates (Na2CO3), chlorides (MgCl2, NaCl, CaCl·H2O), sulfur and sulfur-containing compounds (S8, Na2S), CaF2, and red mud (Table 8). The reducing agents employed can be solid fuels (coal, coke, graphite, biomass char), mixed gases (such as CO/CO2), or hydrogen etc.
| Ore type | Nation/Area | Ni% | Tfe% | Reducing Agents | Additives | Reduction temperature | Reduction time | Nickel grade/recovery | Iron grade/recovery | Source |
| Limonite | Sulawesi | 1.59 | 42.01 | Sub-bituminous coal | CaCO311%+Na2SO4 10% | 700; 1400 | 2 h; 6 h | 18.75; 99.34 | 52.95; 18.78 | Abdul F et al., 2023 |
| Limonite | Indonesia | 1.25 | 55.37 | Sub-bituminous coal | Na2SO4 +CaCO3 | 1400 | 6 h | Grade 21.68 (limestone); Recovery 93.73 (dolomite) | 63.7 (Direct reduction using Na2SO4); - | Widyartha B et al., 2020 |
| Limonite | Sulawesi | 1.59 | 42.01 | Sub-bituminous coal + limestone | Na2S2O3 10% | 700; 1400 | 2 h; 6 h | 14.31; 93.22 | 64.07; 19.02 | Pintowantoro S et al., 2021b |
| saprolite | Indonesia | 1.41 | 24.14 | H2 | Na2S2O3 20% | 1100 | 90 min | 9.97; 99.24 | 44; 35 | Liu S et al.; 2021 |
| Limonite | 1.38 | 56 | Coal 6% | S 4% | 600; 1000 | 1 h; 1 h | 4; 93.2 | 96; - | Elliott R et al., 2015 | |
| Limonite | Sulawesi | 1.59 | 42.01 | Sub-bituminous coal + limestone | S 10% | 700; 1400 | 2 h; 6 h | 13.62; 97.91 | 53.39; 22.18 | Pintowantoro S et al., 2021b |
| Limonite | Philippines | 1.49 | 34.69 | Coal 2% | S 10% | 1200 | 50 min | 7.21; 73.07 | 68.01; 29.43 | Jiang M et al.; 2013 |
| Limonite | Indonesia | 1.16 | 46.55 | Straw charcoal 15% | Red mud 25% | 1250 | 80 min | 1.81; 97.21 | 81.4; 98.87 | Guo X et al.; 2021 |
| Limonite | 1.62 | 14.7 | Anthracite 10% | Red mud 35% | 1200–1300 | 60 min | 4.9; 95.25 | 71; 93.77 | Wang XP et al.; 2018 | |
| Limonite | Sulawesi | 1.38 | 41.06 | Bituminous coal | Na2SO4 | 1250 | 1 h | 7.3; 92 | -; 34 | Nurjaman F et al.; 2021 |
| saprolite | Sulawesi | 1.81 | 18.48 | Bituminous coal | Na2SO4 | 1250 | 1 h | 12.3; 35 | -; 19 | Nurjaman F et al.; 2021 |
| Limonite | Philippines | 1.49 | 34.69 | Coal 2% | Na2SO4 10 % | 1200 | 50 min | 9.87; 90.90 | 72.05; 29.55 | Jiang M et al., 2013 |
| Limonite | Sulawesi | 1.4 | 50.5 | Palm Shell Charcoal 5% | Na2SO4 10 % | 1150 | 1 h | 4.6; 73.2 | 81.9; 35 | Shofi A et al., 2019 |
| Limonite | Indonesia | 1.4 | 50.5 | Anthracite 5% | Na2SO4 15 % | 1150 | 60 min | 15.06; 65 | 60.58; 7.5 | Suharno B. et al., 2021 |
| saprolite | Indonesia | 1.91 | 22.1 | Lignite | Na2SO4 20 % | 1100 | 60 min | 9.48; 83.01 | 79.3; 56.36 | Li G et al., 2012 |
| Limonite | Indonesia | 1.4 | 50.5 | Anthracite 5% | NaCl 15% | 1050 | 60 min | 2; 85.79 | 61.53; 74.67 | Suharno B et al., 2021 |
| Limonite | 1.13 | 35.79 | Coal 10% | NaCl 15% | 1250 | 80 min | 8.15; 97.76 | 64.28; - | Qu GR.et al., 2019 | |
| Limonite | Indonesia | 1.4 | 50.5 | Anthracite 5% | Na2CO3 15% | 1150 | 60 min | 2.18; 67.01 | 55.84; 71.4 | Suharno B et al., 2021 |
| Limonite | Sulawesi | 1.31 | 8.3 | Coal 10% | 10% CaO + 10% CaF2+H3BO3 10% | 1250 | 2 h | 8.59; - | 85.65; - | Zulhan Z and Shalat W, 2021 |
| Limonite | Sulawesi | 1.4 | 16.18 | Coal | CaF2 8% | 1250 | 50 min | 7.1; 84.14 | 68.5; 70.24 | Hang G, 2021 |
| Limonite | Halmahera | 1.29 | 27.02 | Coal 10% | CaSO4 10% | 1100 | 60 min | 4.34; 50.38 | 57.48; 37.58 | Mayangsari W et al., 2018 |
| Limonite | Philippines | 1.49 | 34.69 | Coal 2% | S+Na2O 10 % | 1200 | 50 min | 9.29; 87.29 | 76.22; 30.76 | Jiang M et al., 2013 |
| Limonite | 1.59 | 42.01 | Coal | CaSO4 12% | 1400 | 6 h | 4.13; 91.81 | 27.33; - | Pintowantoro S et al., 2021a | |
| Limonite | Philippines | 0.98 | 48.09 | Limestone +dolomite +slaked lime | 1525 | 45 min | 12.55; 85.65 | 84.61; 10.87 | Wang X et al., 2023 |
Zappala L et al. (2023) reviewed the selective reduction effects of different additives and reducing agents and their combinations, finding that no specific reducing agent (coal, carbon monoxide, hydrogen, coke) significantly outperformed the others. The grade of coal also did not noticeably affect nickel grade and recovery rates. Sulfur and sulfur-containing compounds were identified as the most ideal additives. The addition of calcium oxide to alter the alkalinity of lateritic nickel ore can reduce activation energy; however, further research is needed to determine whether nickel preferentially binds to magnetite during the conversion of hematite to magnetite.
Moreover, the observed reduction in temperature due to the use of additives is only a laboratory result, with no pilot or industrial tests conducted. Additionally, the increased raw material costs from the additives and the savings in operation costs from energy conservation and emissions reduction have yet to be calculated. Sodium-based additives may react with refractory materials, leading to increased maintenance costs, and sulfur-based and chloride additives could generate toxic gases that pollute the environment.
The reduction roasting-ammonia leaching process, also known as the Caron process, was first applied in industrial production for treating lateritic nickel ore at the Nicaro project in Cuba in 1943. The Caron process is typically suitable for processing iron-rich, approximately 1% nickel-bearing limonite ore (Wang CY and Ma BZ, 2020) and saprolite ore with MgO content greater than 10% (Tian QH et al., 2023). The process primarily involves selectively reducing the valuable metal oxides of nickel, cobalt, and some iron in the feed to form an alloy, followed by ammonia leaching. In this stage, nickel, cobalt, and iron enter the leach solution in the form of ammonium complexes (1) – (4). The ammonium ion complex of iron is unstable and transforms into Fe(OH)₃ precipitate under aerobic conditions, entering the slag (5). The leach solution then undergoes sulfide precipitation, ammonia stripping, and calcination to extract nickel and cobalt (Fig. 12).
The principle of ammonia leaching process can be represented as follows (Tian QH et al., 2023):
|
2Ni+O2+2(n−2)NH3+2(NH4)2CO3=2[Ni(NH3)n]CO3+2H2O |
(19) |
|
2Co+O2+2(n−2)NH3+2(NH4)2CO3=2[Co(NH3)n]CO3+2H2O |
(20) |
|
2Fe+O2+2(n−2)NH3+(NH4)2CO3=2[Fe(NH3)n]CO3+2H2O |
(21) |
|
FeO+(n−2)NH3+(NH4)2CO3=[Fe(NH3)n]CO3+H2O |
(22) |
|
4[Fe(NH3)n]CO3+10H2O+O2=4Fe(OH)3↓+4(n−2)NH3+4(NH4)2CO3 |
(23) |
Currently, this process is utilized by Yabulu in Australia, Punta Gorda and Nicaro in Cuba, and Yuanshishan in Qinghai, China (Tables 3 and 7). The main drawbacks of the Caron process are the low recovery rates of nickel and cobalt due to the use of an ammonia-ammonium carbonate leaching system and the reduction degree needs to be strictly controlled by the ammonia atmosphere during selective reduction. Over-reduction of iron can cause difficulties in solid-liquid separation of the slurry and decrease the recovery rate of valuable elements.
The team of Beijing General Research Institute of Mining and Metallurgy conducted metallurgical research on Yuanshishan lateritic nickel ore containing 0.80% nickel. They employed a reduction roasting-ammonia leaching-extraction-magnetic separation process and built a factory with an annual processing capacity of 300000 t of nickel iron ore, which began production in October 2009, producing refined nickel sulfate and iron concentrate, with recovery rates of nickel and iron at 70.70% and 58.85%, respectively (Ruan SF et al., 2015).
Mano ES (2019) analyzed the silicate mineral residue from the Caron process for nickel extraction from the Piaui lateritic nickel ore in Brazil, finding two types of smectite associated with silicate nickel ores. The leaching rate of nickel from trioctahedral smectite rich in Ni and Mg is lower than that from dioctahedral smectite rich in Al and Fe. The lower iron content of the former limited the formation of the Ni-Fe phase, consequently reducing the nickel extraction. This explains why the extraction of nickel from oxidized nickel ores exceeds 90%, while silicate nickel ores yield less than 70%. The Piaui project uses mixed ores, achieving nickel recovery rates of 70‒75%.
de Alvarenga Oliveira V et al. (2019) increased nickel extraction from 3% to 90% by adding 1% NaCl to saprolite ore, hydrogen reduction roasting at temperatures above 850°C, and then ammonia leaching, and attributed this effect to the concentration of nickel chloride formed on the particle surface.
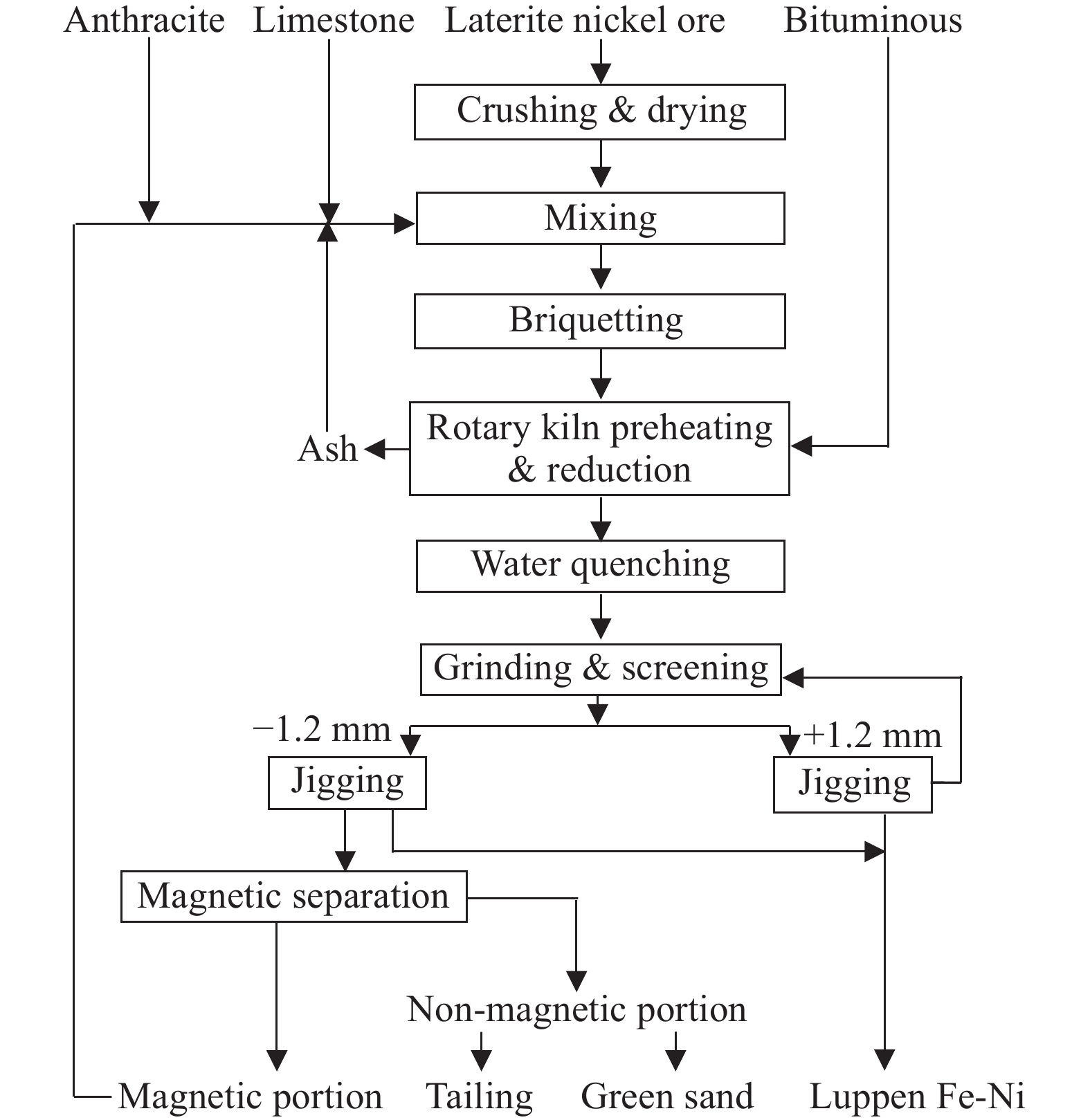
The rotary kiln reduction roasting-magnetic separation process is developed based on the German Krupp-Renn direct reduction ironmaking process. In the 1940s, the Oeyama smelter in Japan used this process to treat limonitic laterites to produce sponge iron, and then shifted to saprolites to produce ferronickel, and achieved mature application by the 1980s (Wang CY and Ma BZ, 2020). This process mainly processes saprolites with a nickel content greater than 1.8%. The process includes: crushing, screening and drying the ore, then mixing it with anthracite and limestone to obtain pellets, and then selectively reducing pellets in a rotary kiln at 1400°C in a semi-molten state to generate ferronickel particles, and then discharging the clinker from the rotary kiln for water quenching, fine grinding, and magnetic separation to obtain ferronickel products (Fig. 13).
The disadvantage of this process is that during the selective reduction process, the limited growth of ferronickel particles leads to low recovery rates of nickel and iron. Hang GH (2020) believes that the aggregation and growth of ferronickel particles is related to the liquid phase sintering, and the low melting point substances wrapped around the ferronickel particles drive the particle migration. To promote the aggregation and growth of ferronickel particles, the rotary kiln needs to reach a higher reduction roasting temperature, which is difficult to operate; on the other hand, since the liquid phase is difficult to control during the production process, it is easy to form rings in the reduction and ferronickel growth areas (Wang S et al., 2021).
To address the shortcomings of the reduction roasting-magnetic separation process, many researchers have explored using different additives to enhance the reduction of laterite nickel ore during rotary kiln roasting (Table 8). Hang GH (2021) studied the effect of CaF2 on selective reduction and found that the addition of CaF2 accelerates the aggregation and growth of ferronickel particles at the boundary between gangue and pores. At the same time, CaF2 reacts with minerals to transform dense forsterite into loose tremolite, increasing mineral activity. The changes in crystal structure and a decrease in melting point increase the migration ability of the metallic phase is conducive to the aggregation and growth of ferronickel particles. In the presence of 8% CaF2, a ferronickel concentrate containing 7.1% nickel and 68.5% iron was obtained, with a Ni recovery rate of 84.14%. When CaF2 is used as an additive, it reduces the surface tension of the newly formed alloy, making the particles easier to aggregate and grow, which benefits the magnetic separation process and affects the grade and recovery rate of the final product. Furthermore, when chlorides are used as additives, the Cl- reacts with H2O in laterite nickel ore to generate HCl, which then reacts with the nickel and iron in the mineral to achieve the purpose of reducing and enriching nickel and iron (Qu T et al., 2020).
Wang L et al. (2018) added SiO2 to briquette to adjust its melting degree during reduction roasting. The results showed that when the mass fraction of SiO2 exceeded 75%, the shape of the briquette did not change, which avoided the adhesion problem with refractory materials and decreased the ring formation of the rotary kiln, but had no obvious effect on the aggregation and growth of ferronickel particles.
Petrus H et al. (2022) compared the performance and kinetics of reducing saprolites using palm kernel shell charcoal and anthracite. The experiment was carried out within a temperature range of 800°C to 1000°C, revealing that the activation energy for the palm kernel shell reducing agent was 10.99–18.19 kJ/mol, significantly lower than the 33.68 kJ/mol for the anthracite reducing agent, indicating that the former is easier to reduce than traditional anthracite.
Ma B et al. (2020) developed a non-molten state metallization reduction (NSMR) technique for the extraction of nickel and iron from saprolite ore in a tube furnace. The recovery of nickel and iron after process optimization increased to 94.1% and 87.4%, respectively. Nickel and iron were metallized in situ before optimization, but there was no migration or aggregation. After optimization, most of the ferronickel solid solutions aggregated and appeared in a banded structure, which was beneficial for magnetic separation.
Although there have been considerable researches on selective reduction in recent years, most of them are in theoretical and experimental stages. Further studies are needed on how to promote nickel and iron reduction, control ring formation, and utilize magnetic separation tailings in the process (Wang S et al., 2021).
In recent years, an increasing number of new methods and technologies have been proposed by researchers, which primarily include bioleaching and chlorination metallurgy. The former utilizes microorganisms to extract nickel with environmental friendliness and low costs. The latter involves using chlorinating agents to convert useful metals in the minerals into chlorides, which are then extracted. Chlorination metallurgy is adaptable, has a short process flow, and offers high comprehensive utilization. However, these new processes are not yet achieved industrial application.
With the tightening of environmental regulations and the decline in ore grades, biohydrometallurgy has provided an alternative for metal extraction since the 1960s. It has been commercially applied to the extraction of copper, gold and silver in South Africa, Russia, Chile, Australia, the United States, China, Myanmar, New Zealand, Peru, Uzbekistan and Ghana (Chaerun SK, 2023). Biohydrometallurgy is to extract valuable metals from ores using bacteria and microorganisms. The principle is to use the oxidation or reduction characteristics of microorganisms to separate the useful metals from minerals into aqueous solutions in ionic or precipitated forms (Li JH et al., 2015). In recent years, efforts have been made to apply this technology for the extraction of nickel and cobalt from low-grade laterite nickel ores (Chaerun SK et al., 2017).
Research on the bioleaching of limonite has found that acidophilic prokaryotes release target metals like nickel and cobalt by catalyzing the oxidation of zero-valent sulfur and reducing Fe(III) and Mn(IV) minerals in limonite (Santos AL et al., 2020) . Although the mechanisms of iron reduction by acidophilic bacteria are still poorly understood (Malik L and Hedrich S, 2022), these studies in laboratory have shown effectiveness for nearly all limonite ores (Santos AL et al., 2020). Research on the bioleaching of the Barro Alto limonite ore in Brazil indicates that “reductive bioleaching” is only applicable to dissolution in MnO-rich mineral phases, such as serpentine and cobaltite, while nickel in goethite remains largely insoluble (Stanković S S et al., 2022).
Hosseini Nasab M et al. (2020) evaluated the bioleaching of cobalt and nickel from limonite by acidophilic heterotrophic bacteria (Delftia acidovorans) and autotrophic bacteria (At. ferrooxidans). The experiments used Iranian Kanshargh laterite nickel ore, which contained 1.74% nickel, 0.14% cobalt, and 40.83% iron. After 3 hours at 90°C, with a stirring speed of 370 rpm and a solid-to-liquid ratio of 0.1, the results indicated that the metabolic products of acidophilic bacteria played a significant role in the bioleaching process. The nickel recovery rates were as high as 83.65% and 80.18% for At. ferrooxidans and Delftia acidovorans supernatants, respectively, with corresponding cobalt recovery rates of 86.93% and 83.94%, and iron dissolution rates of 64.34% and 54.41%. Compared with the direct bioleaching of Delftia acidophilus in an incubator at 30°C and 150 rpm for 30 days, it was found that the extraction rates of nickel and cobalt in indirect bioleaching were 29.84% and 23.75% higher than those in direct bioleaching, respectively. For indirect bioleaching, chemical control has a greater influence on the dissolution rate of limonite than diffusion control. Hosseini Nasab M et al. (2020) investigated the kinetics of two-stage bioleaching of nickel and cobalt from limonite using the metabolic supernatant of Halobiobrachia kushneri, at 90°C after 3 hours, the recoveries of nickel and cobalt reached 58.40% and 60.6%, respectively.
Stanković S et al. (2024) conducted a comparative study of bioleaching and sulfuric acid leaching on Brazil’s Piauí limonite. They simulated heap bioleaching of the laterite in a laboratory-scale column percolator, with a liquid circulative flow rate of 8 mL/min. After one month of bioleaching, the maximum metal extraction rates from the laterite ore were 66% for nickel, 95% for cobalt, 10% for iron, 55% for magnesium, and 89% for manganese. Compared to sulfuric acid leaching, bioleaching achieved higher cobalt extraction rates and lower iron extraction rates, and the pH of the leachate was relatively high, resulting in lower consumption of quicklime and CO2 emissions during neutralization. Carpen HL and Giese EC 2022 were the first to conduct microbial leaching experiments using Burkholderia sp. on Brazil's saprolite ore. The results indicated that Burkholderia sp. exhibited a stronger selective dissolution of nickel than citric acid. The nickel extraction rate was about 87%, and its dissolution rate was higher than that of iron and other metals. This is likely due to Burkholderia sp. participating in the leaching process by metabolizing acids and itself.
Chaerun SK et al. (2017) found that under aerated conditions, Aspergillus niger can effectively produce fungal metabolic acids in a medium containing cassava starch, nitrogen, phosphates and magnesium. The optimal conditions for the metabolic acids were 70 g/L cassava starch, 1% w/v (NH4)2SO4, 0.1% w/v KH2PO4, and 0.25 g/L MgSO4. The metabolic acid production rate from cassava starch surpassed that of molasses, achieving a pH of 1.4 after 16 days. In indirect bioleaching experiments at 95°C, with a stirring speed of 400 rpm, a particle size of −177±149μm for the saprolite ore, a solid-liquid ratio of 4.65 g/mL, and a duration of 4 hours, the maximum nickel extraction rate reached 88.9%, while the magnesium extraction rate after 24 hours was only 1.5%.
The advantages of biometallurgy for laterite nickel ores include being environmentally friendly and having strong selectivity. However, the drawbacks include slow reaction rates, long production cycles, complex processes, and poor stability. As a result, bioleaching processes for laterite nickel ores are currently mainly at the laboratory research stage, and further breakthroughs in biotechnology, such as genetic engineering, are needed for industrial application.
Chlorination metallurgy is a method that involves mixing ores with chlorinating agents (such as Cl2, HCl, NH4Cl, CaCl2, etc.) to react under specific conditions, converting useful metals in the minerals into chlorides, which are then extracted. This method is primarily used for processing rare metals (Li JH et al., 2015). As for nickel extraction from laterite ores, some progress has been made, such as chlorination roasting, chlorination segregating, and chlorination leaching. However, these processes are still in the research and industrial testing stages (Li JH et al. 2016; 2018; Xiao J et al., 2020).
The main process of chlorination roasting involves mixing a chlorinating agent with the nickel laterites, under specific conditions, to convert metal oxides into chlorides, which are then leached with water or other solvents to extract metal ions; alternatively, vaporizing chlorides can be condensed and recovered to obtain metal ions. Li JH et al. (2016) selected a mixture of NaCl and MgCl2·6H2O (in a mass ratio of 0.4) as chlorinating agents to process the nickel laterites mainly composed of garnierite and limonite from Yunnan, China. The sample particle size was −0.074 mm, at 900°C, chlorination roasting 1.5 hours. The extractions for Ni, Co, Mn, Fe, and Mg were 87%, 58%, 53%, 3.2%, and 5.4%, respectively, indicating that chlorination roasting can selectively leach Ni, Co, and Mn, with the Ni/Fe and Ni/Mg ratios in the leachate increasing by 15 and 8 times, respectively.
Chlorination segregation utilizes the characteristics of low melting point and high volatility of metal chlorides, along with the differences in the formation and properties of various metal chlorides. The nickel laterites are calcined with a carbonaceous reducing agent and chlorinating agent in a neutral or weakly reducing atmosphere, allowing valuable metal chlorides to volatilize and subsequently reduce to elemental or metallic particles on the surface of carbon particles, which are then concentrated through magnetic separation. Xiao J et al. (2020) mixed 15% calcium chloride, 15% coke, and 30% iron concentrate with the saprolite ores containing 0.72% nickel and 8.65% iron from Yunnan, roasting at 1100°C for 90 minutes, resulting in a nickel-iron concentrate containing 16.16% Ni and 73.67% Fe, with a nickel recovery rate of 90.33%.
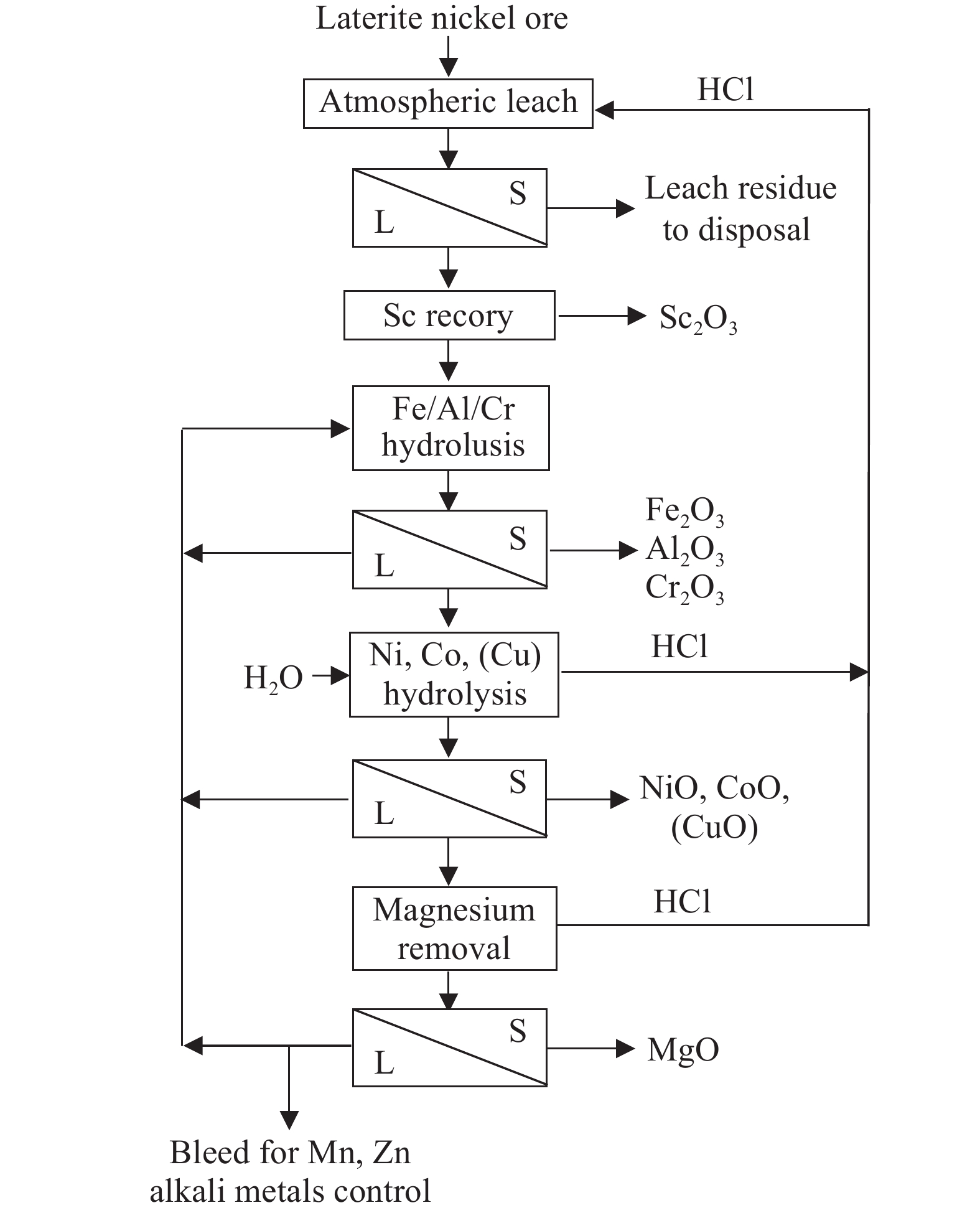
Chlorination leaching is a process using hydrochloric acid or chlorides as the leaching solution. Fan C et al. (2010) employed hexahydrate aluminum chloride as the chlorinating agent to selectively chlorinate saprolites at 300°C–500°C, followed by water leaching at 80°C. Under optimal experimental conditions, the extractions for nickel, cobalt, and iron were 91%, 90%, and 4%, respectively. Li JH et al. (2018) used an ammonium chloride solution as the chlorinating agent, with 2 mol/L HCL, 3 mol/L ammonium chloride, a liquid-to-solid ratio of 6∶1 (mL/g), at 90°C for 90 minutes, resulting in extractions of 87.7% for nickel, 75.1% for cobalt, 95.6% for manganese, and only 21.1% for iron, indicating that chloride ions facilitate garnierite dissolution. Wang YY et al. (2023) carried out a research on the selective hydrolytic extraction of nickel and cobalt from limonite and saprolites using hydrochloric acid. The results indicated that the optimal conditions for chlorination leaching of limonite were a mass ratio of acid to ore of 5∶4, a liquid-to-solid mass ratio of 4∶1, and a reaction temperature of 100°C, achieving extractions of 99.6% for nickel, 100% for cobalt, and 96.9% for iron. Then the leaching solution was mixed with concentrated hydrochloric acid in a volume ratio of 1:4 which was used to leach saprolites under conditions of a liquid-to-solid ratio of 1∶1, 150°C for 90 minutes, the extractions for nickel and cobalt reached 84.9% and 100%, respectively.
Neomet Technologies Inc. has developed the Neomet Chlorination Process, which is a closed-loop chlorination leaching and acid recycling system. The reactions occur in an Atmospheric Autoclave, enabling the recovery of valuable metals from laterite nickel ore, as shown in Fig. 14 (Harris B and White C, 2011, 2013). The results of laboratory and pilot experiments show that after mixing and drying laterite nickel ore from different layers, 800 kg HCl (100%) is added to each ton of dry material, and the reaction is carried out in a 5-stage reactor system at 90°C for 60 minutes each. Valuable metals such as nickel, cobalt, and iron are almost 100% leached, and a 3-stage reactor can achieve a very high leaching rate. Preliminary economic calculations show that the capital cost of this process is only 30% of HPAL.
The chlorination metallurgy process has a lower grade requirement of laterite nickel ore, which reduces resource waste. Additionally, the short process flow, high separation efficiency and multiple metals recovery make the process with a higher comprehensive utilization value. However, the high corrosive to equipment will increase maintenance costs and the challenges in process control and the lack of mature technology are shortcomings that still need to be addressed.
The environmental impacts of nickel extraction from laterites mainly manifest in terms of energy and water resource consumption, greenhouse gas emissions, and pollution of water resources and soil etc. Life cycle assessment (LCA) is a tool used to address the potential environmental impacts of a product throughout its life cycle (ISO 14040, 2006). It has been widely applied in the global metal industry to promote the sustainable development of metal-containing products (Mistry M et al., 2016; Bai YY et al., 2022).
The Nickel Institute (NI) has established a potential environmental impact indicator system for the life cycle assessment of nickel and nickel iron, from mining to refining. The evaluation results for 2023 show that the results of different nickel products for the six key environmental impact indicators of global warming potential, acidification potential, primary energy demand, eutrophication potential, photochemical ozone generation potential and water resource consumption are shown in Table 9. Table 9 illustrates that the environmental impact of producing ferronickel from laterite nickel ore is significantly higher than that of other products. Throughout the entire life cycle of nickel production, the primary extraction is the main contributors to primary energy demand and global warming potential, accounting for 70% and 65% of the total nickel production process, respectively, while electricity consumption accounts for 35% of primary energy demand and 48% of greenhouse gas emissions (Table 10; Mistry M et al., 2016).
| 1 kg Class 1 Ni | 1 kg Ni in FeNi (27% Ni in FeNi) | 1 kg NiSO4 | |
| Global Warming Potential (GWP) [kg CO 2 eq.] | 13 | 45 | 4 |
| Acidification Potential [kg SO 2 eq.] | 1.4 | 0.17 | 0.26 |
| Primary Energy Demand (PED) [MJ] | 236 | 592 | 68 |
| Eutrophication Potential [kg Phosphate eq.] | 5.2E-03 | 0.016 | 1.5E-03 |
| Photochemical Ozone Creation Potential [kg Ethene eq.] | 0.055 | 0.010 | 0.010 |
| Blue water consumption [kg] | 106 | 924 | 49 |
| Impact category (unite) | Significant process (% of impact category) | Significant contributor to the process (% of process) | Significant flow (% of impact category) |
| PED (MJ) | Primary extraction (70%) | Electricity (35%) | Crude oil (43%) natural gas (17%) |
| GWP (kg CO2 -equivalents) | Primary extraction (65%) | Electricity (48%) | Carbon dioxide (–100%) |
Wei W et al. (2020) argue that nickel LCA methods often ignore differences in ore type, grade, product, and operating conditions, whereas process modeling based on the laws of mass and energy conservation can address the shortcomings of LCA. The analysis results from mass-energy conservation modeling indicate that if 100% hydroelectric power is used in ferronickel production, energy consumption and carbon emissions will decrease by 37% and 64%, respectively, compared to using coal. Conversely, when the nickel grade decreases from 2.5% to 0.5%, carbon emissions from ferronickel production will increase by 43%. In a mixed energy context, the energy consumption per ton of nickel for three nickel products processed from laterite ore are NiO (485 GJ), FeNi (309 GJ) and NPI (598 GJ), respectively, and the carbon emissions are NiO (40tCO2 -eq/t), FeNi (18tCO2 -eq/t) and NPI (69tCO2 -eq/t), respectively, which are significantly higher than the 174 GJ and 14tCO2 -eq/t of nickel metal produced from sulfide nickel ore (Table 11) . If factors such as thermal recycling in the production process are excluded, the energy consumption per ton of nickel for the three nickel products will decrease by 36% for NiO, 44% for FeNi and 36% for NPI, and carbon emissions will decrease by 40% for NiO, 44% for FeNi and 17% for NPI.
| Ore type | Nickel sulfide ore | Nickel laterite | Nickel laterite | Nickel laterite | |
| Product type | Nickel metal 100%Ni |
NiO (76% Ni) | FeNi (35% Ni) | NPI (10% Ni) | |
| The most energy-consuming sub- processes | Refining | Smelting | Calcination | Smelting | |
| Energy consumption contribution of the sub- processes to the process (%) | 44 | 45 | 44 | 67 | |
| Carbon emissions contribution of the sub- processes to the process (%) | 38 | 45 | 55 | 73 | |
| Carbon emission sources and energy of the sub- processes | Electricity | Fossil fuels and reducing agents | Fossil fuels and reducing agents | Fossil fuels and reducing agents | |
| Mixed energy | 60% coal | 49% oil | 70%hydro | 49% coal | |
| Energy consumption per ton of nickel product (GJ) | 174 | 370 | 110 | 60 | |
| Energy consumption per ton of nickel metal (GJ) | 174 | 485 | 309 | 598 | |
| Carbon emissions per ton of nickel product (tCO2 -eq) | 14 | 30 | 6 | 7 | |
| Carbon emissions per ton of nickel metal (tCO2 -eq) | 14 | 40 | 18 | 69 | |
| 100% hydro-power electricity | |||||
| Energy consumption per ton of nickel product (GJ) | 127 | 270 | 102 | 59 | |
| Carbon emissions per ton of nickel product (tCO2 -eq) | 7 | 17 | 4.9 | 7 | |
| Deduction for recycling, etc. | |||||
| Energy consumption per ton of nickel product (GJ) | 116 | 235 | 61 | 39 | |
| Energy consumption per ton of nickel metal (GJ) | 116 | 309 | 172 | 385 | |
| Carbon emissions per ton of nickel product (tCO 2 -eq) | 6 | 18 | 4 | 6 | |
| Carbon emissions per ton of nickel metal (tCO 2 -eq) | 6 | 24 | 10 | 57 | |
The analysis above indicates that using clean energy can significantly reduce the environmental impact of nickel extraction from laterite ores. Additionally, process improvements such as thermal energy recovery during production and replacing blast furnaces with electric furnaces can also mitigate environmental effects. Khoo JZ et al. (2017) compared the energy consumption, carbon emissions, water resource usage, and biotoxicity of three nickel extraction processes—HPAL, RKEF, and direct nickel method—when producing one ton of stainless steel. Under the assumption that all processes use coal-fired power and without considering ore grades, the results showed that RKEF had the highest carbon emissions and biotoxicity impact, while HPAL had the lowest. Conversely, HPAL consumed the most water, while the direct nickel method consumed the least. After comprehensive evaluation, the direct nickel method was found to have the highest sustainability in nickel resource utilization, providing a new source of nickel oxide for stainless steel production, with higher production efficiency since 35 t of laterite ore are required to produce one ton NiO.
In addition to the significant and direct environmental impacts of energy consumption and carbon emissions, the potential effects of toxic leachable metals in slag and leach residues on soil and water resources should not be overlooked (Table 12). Estimates suggest that producing one ton of nickel metal via pyrometallurgical processes generates 6–16 t of slag, with global annual slag production around 150×106 t, while producing one ton of nickel metal via HPAL produces 150–200 t of acidic tailings, which, if not properly managed, can leach heavy metals and harmful substances like acids and alkalis into the soil and groundwater, posing potential risks to local ecosystems and human health (Bartzas G et al., 2021). Moreover, Taylor MP et al. (2023) used bees as biological markers of environmental pollution to investigate the impact of the Doniambo nickel smelter, operating since 1910 and located 2 km from the capital of New Caledonia. The results showed that the concentrations of potentially toxic trace elements such as cobalt, chromium, and nickel in the bees decreased with increasing distance from the smelter, indicating the potential risks posed by the nickel smelter to the surrounding environment and human health.
| Content of heavy metal(loid)s (mg/kg) in slag and leaching residue from Ni laterite | |||||||
| element | Slag from Ni laterites | Leaching residue from Ni laterite | |||||
| Min | Max | Average | Min | Max | Average | ||
| As | 3.3 | 26.2 | 11.8 | 25 | 425 | 300 | |
| Co | 40 | 220 | 115 | 10 | 890 | 218 | |
| Cr | 6340 | 8000 | 6848 | 4600 | 17400 | 9150 | |
| Cu | 6.23 | 1157 | 399 | 36 | 320 | 127 | |
| Mn | 2650 | 3500 | 3007 | 100 | 8150 | 2558 | |
| Ni | 740 | 3200 | 1303 | 330 | 3400 | 1763 | |
| Zn | 85 | 1162 | 357 | 130 | 480 | 300 | |
| Cd | 0.25 | 4.58 | 2.4 | ||||
| Pb | 39 | 300 | 195 | ||||
| Sb | 26.2 | 79.2 | 45.8 | ||||
| V | 65.9 | 84.8 | 75.2 | ||||
The economics of nickel extraction from laterite nickel ore involves many factors, including ore grade, extraction process, energy cost, labor cost, reagents and transportation, market demand, environment and policy etc. Ore grade, market demand, and infrastructure determine the extraction process and product type, which in turn influence investment costs. Operation costs are determined by factors such as ore grade, energy, labor, reagents, and transportation costs. Market demand, environmental considerations, and policies influence prices, while costs and prices ultimately determine the economic viability of mining projects. The economic evaluation of nickel extraction from laterite ores requires a detailed cost-benefit analysis based on the specific circumstances of a project. The global economic evaluation results of major operating laterite nickel projects based on S&P are presented in Table 13.
| Processing Method | Production Forms | Head Grade Ni% | CapEx(k$/ t)Ni Capacity | OpEx ($/t) | Production Capacity(kt)Ni | Recovery Rate Ni% |
| HPAL | MHP/MSP | 1–1.8 | 56 | 15198 | 28–60 | 83–90 |
| RKEF | FeNi | 1.31–2.2 | 43 | 16278 | 10–55 | 77–90 |
| Smelting | Matte | 1.69 | 1 | 14583 | 80 | 88 |
| AL | Hydroxide | 1.3 | – | 12565 | 1 | 85 |
| Caron | Concentrate | 1.2 | 19 | 17611 | 30 | 70 |
| HL | Hydroxide | Saprolite-limonite | 20–33 | 4889–6667 | 10–60 | 86 |
| OESB | Matte | Saprolite-limonite | 22 | 7414–8081 | 30 | 93 |
| Note: HL data is from Oxley A et al., (2016), OESB production cost data is from Chen XG et al., 2018, and the rest data are from S&P laterite nickel ore economic model | ||||||
The RKEF usually has a designed capacity of 10000 t to 55000 t, with a capital cost of approximately 43000pertonofnickelmetalandanoperationalcostofabout16000 per ton, and the total nickel recovery rate is 77%–90%. Currently, around 80% of laterite nickel ore is produced using RKEF, primarily for the stainless steel sector (Nurjaman F, 2020). In contrast, the HPAL generally has a designed capacity of 28000 t to 60000 t, with a capital cost of approximately 56000pertonofnickelmetalandanoperationalcostofabout15000 per ton, achieving a total nickel recovery rate of 83% – 90%, mainly for the clean energy power battery sector. Additionally, a small number of projects use sulfidation smelting nickel matte processes, atmospheric acid leaching processes, or reduction roasting-ammonia leaching processes, their detail costs in Table 13. Overall, the cost differences between the two mainstream processes are not significant; the main distinctions lie in the product types, end uses, and carbon emissions. The choice of process primarily depends on ore type, market demand, and carbon taxes. As demand for stainless steel weakens and the demand for nickel sulfate in power batteries increases, along with the implementation of carbon taxes, the economic viability of the HPAL process is expected to improve. Although the technology route for converting ferronickel to high nickel matte for nickel sulfate production has been established, it is only economically viable when the price difference between nickel sulfate and ferronickel exceeds $17000 per ton (Zhang ZF et al., 2022).
Oxley A et al. (2016) suggested that in the pyrometallurgical process of laterite nickel ore, the ore must meet very specific standards, namely, Fe/Ni, Ni/Co and SiO2/MgO ratios of 12, 40 and 1.9 respectively, to achieve good recovery and product grade. These standards can be relaxed, but this may lead to ineffective operations due to declining recovery rates and product quality. Newly constructed ferronickel smelting plants typically require a nickel grade exceeding 1.8%, with an initial grade above 2%, to expect capital returns within five years. Additionally, the Fe/Ni, Ni/Co and SiO2/MgO ratios must be <12, >30 and <1.9 respectively for commercial economic operation.
Indonesia’s abundant laterite nickel resources and relatively low operation costs enable it to better withstand fluctuations in nickel prices, thereby influencing the economics of global primary nickel production. With the implementation of the export ban on unprocessed ore in 2020, Indonesia's primary nickel output has increased from 383000 t in 2019 to 1363000 t in 2023, and S&P forecasts that by 2027, its output will account for 44% of the global total (Fig. 15), A new supply landscape for primary nickel is emerging.
At the same time, with the advancement of the “dual carbon” goals, the consumption pattern for primary nickel is also changing. It is predicted that by 2030, the demand for nickel sulfate will account for 30% of total demand (Zhang ZF et al., 2022). The current production routes for nickel sulfate mainly include the HPAL, RKEF and OESB (Table 14). Compared to these three routes, the HPAL has a higher investment intensity but offers advantages in operation costs and environmental benefits. While the pyrometallurgical route for converting ferronickel to high nickel matte has slightly lower investment intensity, the high operation costs and inherent issues of high energy consumption and emissions reduce its competitiveness. The OESB still needs practical validation.
| Economic and environmental indicators | Ferronickel sulfidation blowing | Oxygen-enriched side blowing | High pressure acid leaching |
| Ni Capital intensity | 12 | 8.1 | 12–20 |
| Cash cost + maintenance expenditure of nickel sulfate (k$/t) | 13–14 | 11–12 | 9.7–10 |
| Construction period (year) | 2 | 1–2 | 2–3 |
| Raw material requirements | Humus soil type | Various types | Limonite type |
| Tailings (t slag/t Ni) | 6–16 | – | 150–200 |
| Energy consumption | 592 | – | 272 |
| Carbon emissions | 45 | – | 19 |
As of 2022, Indonesia has already commissioned HPAL capacities of 230000 t, with plans to add 4.575×106 t by 2026. Additionally, there are plans to start up 42000 t of intermediate for further refining into nickel sulfate, while the proposed capacities for ferronickel and high nickel matte are only 79000 t (Heijlen W and Huhayon C, 2024). This indicates that Indonesia primarily aims to meet the increasing global nickel demand and changes in consumption structure by increasing its HPAL processing capacity.
With the development of the new energy industry, nickel, as an important energy metal, has been designated as a strategic mineral by many countries and is receiving increasing global attention. Due to the depletion of sulfide nickel resources and changes in nickel consumption patterns, laterite nickel ore is gradually becoming the main source of primary nickel. However, its low grade, complex composition, and challenges in separation and enrichment make research into nickel extraction processes from laterite ores particularly significant for meeting new low-carbon consumption demands. This paper reviews the research progress on nickel extraction from laterite ores using hydrometallurgical, pyrometallurgical, and combined processes, focusing on the energy consumption, carbon emissions, environmental impacts, and economic viability of mainstream processes. The main conclusions are:
(i) The HPAL process is expected to become the primary method for nickel extraction from laterite ores, as it meets the growing nickel demand in the new energy industry while exhibiting lower energy consumption and carbon emissions, high nickel recovery rates, and the ability to comprehensively recover valuable metals such as cobalt and iron. It can efficiently utilize low-grade laterite nickel ores, but challenges remain regarding high investment intensity, substantial solid and liquid waste, and process complexity.
(ii) The most widely used pyrometallurgical process is RKEF, which primarily employs high grade saprolites to produce ferronickel, serving as a major source of nickel for stainless steel. The RKEF process offers high nickel recovery rates and minimal harmful impurities in ferronickel, with a short production flow and high efficiency. However, it still faces issues such as high energy consumption, significant slag generation, and elevated operation costs. Although it has achieved flexible conversion to high nickel matte and subsequently to nickel sulfate to meet the new energy sector’s nickel demand, its investment intensity, operation costs, and construction periods are all higher than those of the oxygen-enriched side-blown process. Furthermore, the oxygen-enriched side-blown process has a wider material adaptability and flexible control over nickel grades of the products, although production efficiency remains to be evaluated, its development prospects are promising.
(iii) The combined pyrometallurgical and hydrometallurgical processes leverage the advantages of both methods. The use of additives lowers the temperature for selective reduction, which not only saves energy and reduces emissions but also decreases operation costs. However, since selective reduction is still in the experimental phase, the increase in raw material costs due to additives and the reduction in operation costs need to be evaluated for economic viability in future industrial applications. Bioleaching processes are environmentally friendly and selective, but their slow reaction speed and poor stability mean that breakthroughs in biotechnologies, such as genetic engineering, are still needed for industrial production. Chlorination metallurgy offers broad material selectivity, allowing for the recovery of multiple metals, including nickel, cobalt, iron, and magnesium, with a high degree of resource utilization. It has a short process flow and high separation efficiency, but the technology is not yet mature.
(iv) Throughout the entire life cycle of nickel production, the primary energy demand and global warming potential from the nickel extraction stage account for 70% and 65% of the entire nickel production process, respectively. Therefore, optimizing the nickel extraction process is crucial. The use of hydrometallurgy and clean energy can significantly reduce the environmental impact of extracting nickel from laterite ores. In a scenario where stainless steel remains the primary consumer, the direct nickel method offers the highest sustainability for nickel resources and can provide new nickel oxide raw materials for stainless steel production. As the demand for nickel in the renewable energy sector increases, the economic competitiveness of high-pressure acid leaching and oxygen-enriched side-blowing processes for producing nickel sulfate becomes more pronounced.
Each nickel extraction process from laterite ores has its advantages and room for improvement. Therefore, it is essential to strengthen research on nickel extraction processes to save energy, reduce emissions, and meet different nickel demands. Additionally, it is appropriate to conduct comparative studies on the environmental impacts of nickel used in batteries for transportation and energy storage, as well as the environmental impacts of nickel metal production, to clarify the economic viability and competitiveness of nickel extraction.
Zhen-fang Zhang, Wei-bo Zhang, Zhen-guo Zhang and Xiu-fa Chen conceived of the presented idea. All authors discussed the results and contributed to the final manuscript.
The authors declare no conflicts of interest.
This research was jointly supported by the China Geological Survey Project (DD20211404) and the Natural Science Foundation of Inner Mongolia Autonomous Region (2019LH05028). Special thanks to Yue-ming Wang for his map creation and review of the references. Many thanks to David Zhang for polishing the text. Thank you to both reviewers for your valuable comments and suggestions.
|
Abdul F, Firdausi S, Widyartha AB, Setiyorini Y, Pintowantoro S. 2023. The role of limestone in enhancing selective reduction of nickel in the carbothermic reduction of laterite nickel. Transactions of the Indian Institute of Metals, 76(8), 2211–2219. doi: 10.1007/s12666-023-02938-w.
|
|
Andersson P. 2020. Chinese assessments of “critical” and “strategic” raw materials: Concepts, categories, policies, and implications. The Extractive Industries and Society, 7(1), 127–137. doi: 10.1016/j.exis.2020.01.008.
|
|
Bai YY, Zhang TZ, Zhai YJ, Jia YK, Ren K, Hong JL. 2022. Strategies for improving the environmental performance of nickel production in China: Insight into a life cycle assessment. Journal of Environmental Management, 312, 114949. doi: 10.1016/j.jenvman.2022.114949.
|
|
Bartzas G, Tsakiridis PE, Komnitsas K. 2021. Nickel industry: Heavy metal(loid)s contamination - sources, environmental impacts and recent advances on waste valorization. Current Opinion in Environmental Science & Health, 21, 100253. doi: 10.1016/j.coesh.2021.100253.
|
|
Brown T. 2018. Measurement of mineral supply diversity and its importance in assessing risk and criticality. Resources Policy, 58, 202–218. doi: 10.1016/j.resourpol.2018.05.007.
|
|
Carpen HL, Giese EC. 2022. Enhancement of nickel laterite ore bioleaching by Burkholderia sp. using a factorial design. Applied Water Science, 12(8), 181. doi: 10.1007/s13201-022-01704-5.
|
|
Chaerun SK, Winarko R, Yushandiana F, Veteran UPNU. 2023. Biohydrometallurgy: Paving the way for a greener future of mineral processing in Indonesia. Current Research on Biosciences and Biotechnology, 5(1), 299–307. doi: 10.5614/crbb.2023.5.1/8kiz3aoe.
|
|
Chaerun SK, Sulistyo RS, Minwal WP, Mubarok MZ. 2017. Indirect bioleaching of low-grade nickel limonite and saprolite ores using fungal metabolic organic acids generated by Aspergillus Niger. Hydrometallurgy, 174, 29–37. doi: 10.1016/j.hydromet.2017.08.006.
|
|
Chen XG, Pei ZY, Dai WB, Xu XF, Cui M, Yu Y, Wang SX. 2018. Application and prospect of side-submerged combustion smelting (SSC) process for nickel laterite. China Nonferrous Metallurgy, 47(6), 1–7 (in Chinese with English abstract). doi: 10.19612/j.cnki.cn11-5066/tf.2018.06.001.
|
|
Chen XG, Qi YF. 2023. Experimental research by side-blown reducing-matting smelting of laterite nickel ore. Energy Saving of Nonferrous Metallurgy, 39(1), 33–39 (in Chinese). doi: 10.19610/j.cnki.cn11-4011/tf.2023.01.005.
|
|
Cui YL, Yang XS, Jiang YG, Chen YX. 2013. Metallogenic conditions and prospecting indicators for the lateritic nickel deposits. Acta Mineralogica Sinica, 33(4), 449–455 (in Chinese with English abstract). doi: 10.16461/j.cnki.1000-4734.2013.04.002.
|
|
Dalvi AD , Bacon WG, Osborne RC. 2004. The past and the future of nickel laterites. PDAC 2004 International Convention, Trade Show & Investors Exchange, 1–27.
|
|
de Alvarenga Oliveira V, de Jesus Taveira Lana R, da Silva Coelho HC, Brigolini GJS, dos Santos CG. 2020. Kinetic studies of the reduction of limonitic nickel ore by hydrogen. Metallurgical and Materials Transactions B, 51(4), 1418–1431. doi: 10.1007/s11663-020-01841-9.
|
|
de Alvarenga Oliveira V, dos Santos CG, de Albuquerque Brocchi E. 2019. Assessing the influence of NaCl on the reduction of a siliceous laterite nickel ore under caron process conditions. Metallurgical and Materials Transactions B, 50(3), 1309–1321. doi: 10.1007/s11663-019-01552-w.
|
|
Dong B, Tian QH, Xu ZP, Li D, Wang QA, Guo XY. 2023. Advances in clean extraction of nickel, cobalt and lithium to produce strategic metals for new energy industry. Materials Reports, 37(22), 127–141 (in Chinese with English abstract). doi: 10.11896/cldb.22090071.
|
|
Duman BÖ, Can İB. 2022. Effects of staged-addition of acid on high NiCo recovery and low scale formation in HPAL of a lateritic ore. Hydrometallurgy, 213, 105935. doi: 10.1016/j.hydromet.2022.105935.
|
|
Elliott R, Rodrigues F, Pickles CA, Peacey J. 2015. A two-stage thermal upgrading process for nickeliferous limonitic laterite ores. Canadian Metallurgical Quarterly, 54(4), 395–405. doi: 10.1179/1879139515y.0000000009.
|
|
European Commission, Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs, Grohol, M., Veeh, C., Study on the critical raw materials for the EU 2023 – Final report, Publications Office of the European Union, 2023, doi: 10.2873/725585
|
|
Fan CL, Zhai XJ, Fu Y, Chang YF, Li BC, Zhang TA. 2010. Extraction of nickel and cobalt from reduced limonitic laterite using a selective chlorination–water leaching process. Hydrometallurgy, 105(1–2), 191–194. doi: 10.1016/j.hydromet.2010.08.003.
|
|
Fraser, J., Anderson, J., Lazuen, J., et al. Study on future demand and supply security of nickel for electric vehicle batteries, Publications Office of the European Union, Luxembourg, 2021, ISBN 978-92-76-29139-8, doi: 10.2760/212807, JRC123439.
|
|
Guo H, Fu HK, Jing QX, Wu LJ, Liang WC. 2020. Atmospheric leaching of Ni, Co and Fe in laterite nickel ore using sulfuric acid. Hydrometallurgy of China, 39(3), 190–193,202 (in Chinese with English abstract). doi: 10.13355/j.cnki.sfyj.2020.03.004.
|
|
Guo YS, Luo YF. 2013. Geology and exploration of lateritic nickel deposits in China and Southeast Asia. Beijing, Geological Press, 4–21(in Chinese)
|
|
Guo Q, Qu JK, Han BB, Zhang PY, Song YX, Qi T. 2015. Innovative technology for processing saprolitic laterite ores by hydrochloric acid atmospheric pressure leaching. Minerals Engineering, 71, 1–6. doi: 10.1016/j.mineng.2014.08.010.
|
|
Guo XS, Li ZY, Han JC, Yang D, Sun TC. 2021. Study of straw charcoal as reductant in co-reduction roasting of laterite ore and red mud to prepare powdered ferronickel. Mining, Metallurgy & Exploration, 38(5), 2217–2228. doi: 10.1007/s42461-021-00466-z.
|
|
Hang GH. 2020. Fundamental research on preparation offerronickel from a low-grade typicalsaprolitic laterite ore. Wuhan, Wuhan University of Science and Technology, Ph. D thesis, 1–116 (in Chinese with English abstract). doi: 10.27380/d.cnki.gwkju.2020.000564.
|
|
Hang GH, Xue ZL, Wu YJ, Zhang B. Effect of CaF2 on the aggregation and growth of ferronickel particles in the self-reduction of Nickel laterite ore. Metallurgical Research & Technology, 118(4), 407. doi: 10.1051/metal/2021050.
|
|
Harris, B., & White, C. 2011. Recent developments in the chloride processing of nickel laterites. Proc. 2nd ALTA Ni–Co–Cu Conf., Perth, Australia.
|
|
Harris, B., & White, C. 2013. Further development of the chloride process for base and light metals: Recent miniplant and first pilot plant data. Proc. 4th ALTA Ni–Co–Cu Conf., Perth, Australia.
|
|
Heijlen W, Duhayon C. 2024. An empirical estimate of the land footprint of nickel from laterite mining in Indonesia. The Extractive Industries and Society, 17, 101421. doi: 10.1016/j.exis.2024.101421.
|
|
Hosseini Nasab M, Noaparast M, Abdollahi H, Ali Amoozegar M. 2020. Kinetics of two-step bioleaching of Ni and Co from iron rich-laterite using supernatant metabolites produced by Salinivibrio kushneri as halophilic bacterium. Hydrometallurgy, 195, 105387. doi: 10.1016/j.hydromet.2020.105387.
|
|
IEA 2023. Critical Minerals Market Review 2023, IEA, Paris https://www.iea.org/reports/critical-minerals-market-review-2023, License: CC BY 4.0
|
|
IEA, 2021. GHG emissions intensity for class 1 nickel by resource type and processing route, IEA, Paris https://www.iea.org/data-and-statistics/charts/ghg-emissions-intensity-for-class-1-nickel-by-resource-type-and-processing-route, IEA. Licence: CC BY 4.0
|
|
ISO 14040, 2006. Environmental Management-Life Cycle Assessment-Principles and Frame Work. British Standards Institution, London. https://www.iso.org/standard/37456.html.
|
|
Jiang M, Sun TC, Liu ZG, Kou J, Liu N, Zhang SY. 2013. Mechanism of sodium sulfate in promoting selective reduction of nickel laterite ore during reduction roasting process. International Journal of Mineral Processing, 123, 32–38. doi: 10.1016/j.minpro.2013.04.005.
|
|
Keskinkilic E. 2019. Nickel laterite smelting processes and some examples of recent possible modifications to the conventional route. Metals, 9(9), 974. doi: 10.3390/met9090974.
|
|
Khoo JZ, Haque N, Woodbridge G, McDonald R, Bhattacharya S. 2017. A life cycle assessment of a new laterite processing technology. Journal of Cleaner Production, 142, 1765–1777. doi: 10.1016/j.jclepro.2016.11.111.
|
|
König U. 2021. Nickel laterites—Mineralogical monitoring for grade definition and process optimization. Minerals, 11(11), 1178. doi: 10.3390/min11111178.
|
|
Li GH, Luo J, Jiang T, Peng ZW, Rao MJ, & Zhang YB. 2017. A method for reducing energy consumption in the production of nickel-iron alloy using the RKEF process. CN201711388806.2(in Chinese).
|
|
Li JH, Xu ZF, Gao Y, Li DS, Liu Y, Zhao CD. 2019. Selectively leaching valuable metals from laterite nickel ore by ammonium chloride. The Chinese Journal of Nonferrous Metals, 29(5), 1049–1057 (in Chinese with English abstract). doi: 10.19476/j.ysxb.1004.0609.2019.05.18.
|
|
Li JH, Xu ZF, Wang RX. 2019. Fundamental research on chlorination metallurgy technology for lateritic nickel ores. Beijing: Metallurgical Industry Press, 12–17 (in Chinese)
|
|
Li JH, Li YY, Zheng S, Xiong DL, Chen H, Zhang YF. 2015. Research review of laterite nickel ore metallurgy. Nonferrous Metals Science and Engineering, 6(1), 35–40 (in Chinese with English abstract). doi: 10.13264/j.cnki.ysjskx.2015.01.007.
|
|
Li GH, Shi TM, Rao MJ, Jiang T, Zhang YB. 2012. Beneficiation of nickeliferous laterite by reduction roasting in the presence of sodium sulfate. Minerals Engineering, 32, 19–26. doi: 10.1016/j.mineng.2012.03.012.
|
|
Li JH, Li DS, Xu ZF, Liao CF, Liu Y, Zhong B. 2018. Selective leaching of valuable metals from laterite nickel ore with ammonium chloride-hydrochloric acid solution. Journal of Cleaner Production, 179, 24–30. doi: 10.1016/j.jclepro.2018.01.085.
|
|
Li JH, Li YY, Gao Y, Zhang YF, Chen ZF. 2016. Chlorination roasting of laterite using salt chloride. International Journal of Mineral Processing, 148, 23–31. doi: 10.1016/j.minpro.2016.01.007.
|
|
Liu SJ, Yang C, Yang S, Yu ZL, Wang Z, Yan K, Li J, Liu XY. 2021. A robust recovery of Ni from laterite ore promoted by sodium thiosulfate through hydrogen-thermal reduction. Frontiers in Chemistry, 9, 704012. doi: 10.3389/fchem.2021.704012.
|
|
Luo J, Li GH, Rao MJ, Peng ZW, Zhang YB, Jiang T. 2015. Atmospheric leaching characteristics of nickel and iron in limonitic laterite with sulfuric acid in the presence of sodium sulfite. Minerals Engineering, 78, 38–44. doi: 10.1016/j.mineng.2015.03.030.
|
|
Ma BZ, Wang CY, Yang B, Yin F, Zhang YL. 2011. Pilot plant study on pressure leaching of nickel laterite in nitric acid medium. The Chinese Journal of Process Engineering, 11(4), 561–566 (in Chinese with English abstract).
|
|
Ma BZ, Li X, Yang WJ, Hu D, Xing P, Liu B, Wang CY. 2020. Nonmolten state metalized reduction of saprolitic laterite ores: Effective extraction and process optimization of nickel and iron. Journal of Cleaner Production, 256, 120415. doi: 10.1016/j.jclepro.2020.120415.
|
|
Malik L, Hedrich S. 2022. Ferric iron reduction in extreme acidophiles. Frontiers in Microbiology, 12, 818414. doi: 10.3389/fmicb.2021.818414.
|
|
Mano ES, Caner L, Petit S, Chaves AP, Mexias AS. 2019. Ni-smectitic ore behaviour during the Caron process. Hydrometallurgy, 186, 200–209. doi: 10.1016/j.hydromet.2019.04.010.
|
|
Mayangsari W, Prasetyo AB, Prasetiyo P. 2018. Upgrading nickel content of limonite nickel ore through pelletization, selective reduction and magnetic separationProceedings of the 3RD International Conference on Materials and Metallurgical Engineering and Technology (Icommet 2017): Advancing Innovation in Materials Science, Technology and Applications for Sustainable Future, Surabaya, Indonesia. doi: 10.1063/1.5030243.
|
|
Miao Z, Sun NL & Li SL. 2020. Effect of magnesium content on the cost of pressure acid leaching of Ramu laterite nickel ore. China Nonferrous Metals(04), 11–13 (in Chinese with English abstract) . doi: 10.19612/j.cnki.cn11-5066/tf.2020.04.003.
|
|
Mistry M, Gediga J, Boonzaier S. 2016. Life cycle assessment of nickel products. The International Journal of Life Cycle Assessment, 21(11), 1559–1572. doi: 10.1007/s11367-016-1085-x.
|
|
Nickel Institute. Life Cycle Assessment of Nickel Products; Report for Nickel Institue: Toronto, ON, Canada, 2023
|
|
Nurjaman F, Astuti W, Bahfie F, Suharno B. 2021. Study of selective reduction in lateritic nickel ore: Saprolite versus limonite. Materials Today: Proceedings, 44, 1488–1494. doi: 10.1016/j.matpr.2020.11.687.
|
|
Nurjaman F, Bahfie F, Herlina U, Astuti W, Suharno B. 2020. Kajian literatur parameter proses reduksi selektif bijih nikel laterit. Metal Indonesia, 42(2), 63. doi: 10.32423/jmi.2020.v42.63-71.
|
|
Oxley A, Smith ME, Caceres O. 2016. Why heap leach nickel laterites? Minerals Engineering, 88, 53–60. doi: 10.1016/j.mineng.2015.09.018.
|
|
Palovaara P, Pisila S, Sakaranaho M, Lindvall M, Tikka J. 2021. Benchmarking of FeNi electric arc furnace operations for developing furnace design parameters for lateritic ores in the intermediate SiO2/MgO range of between 2.2 and 2.7. Proceedings of the 16th International Ferro-Alloys Congress (INFACON XVI). DOI: 10.2139/ssrn.3926636
|
|
Pandey N, Tripathy SK, Patra SK, Jha G. 2023. Recent progress in hydrometallurgical processing of nickel lateritic ore. Transactions of the Indian Institute of Metals, 76(1), 11–30. doi: 10.1007/s12666-022-02706-2.
|
|
Petrus HTBM, Putera ADP, Warmada IW, Nurjaman F, Astuti W, Prasetya A. 2022. Investigation on saprolitic laterite ore reduction process using palm kernel shell charcoal: Kinetics and phase transformation. International Journal of Technology, 13(3), 565. doi: 10.14716/ijtech.v13i3.4701.
|
|
Pintowantoro S, Muhammad Pasha RA, Abdul F. 2021. Gypsum utilization on selective reduction of limonitic laterite nickel. Results in Engineering, 12, 100296. doi: 10.1016/j.rineng.2021.100296.
|
|
Pintowantoro S, Widyartha AB, Setiyorini Y, Abdul F. 2021. Sodium thiosulfate and natural sulfur: Novel potential additives for selective reduction of limonitic laterite ore. Journal of Sustainable Metallurgy, 7(2), 481–494. doi: 10.1007/s40831-021-00352-4.
|
|
Qu T, Gu XP, Shi L, Luo MY, Wang Q, Lü F, Tian Y, Dai YN. 2020. Rsearch status of development and utilization of garnierite. Materials Reports, 34(S1), 261–267 (in Chinese).
|
|
Qu GR, Zhou SW, Wang HY, Li B, Wei YG. 2019. Production of ferronickel concentrate from low-grade nickel laterite ore by non-melting reduction magnetic separation process. Metals, 9(12), 1340. doi: 10.3390/met9121340.
|
|
Rao MJ, Li GH, Jiang T, Luo J, Zhang YB, Fan XH. 2013. Carbothermic reduction of nickeliferous laterite ores for nickel pig iron production in China: A review. JOM, 65(11), 1573–1583. doi: 10.1007/s11837-013-0760-7.
|
|
Ruan SF, Wang CY, Yin F, Chen YQ, Wang J, Jie XW. 2015. Project design of comprehensive utilization of nickel laterite from Yuanshishan in Qinghai. Nonferrous Metals Engineering, 5(1), 41–45 (in Chinese). doi: 10.3969/j.issn.2095-1744.2015.01.010.
|
|
Santos AL, Dybowska A, Schofield PF, Herrington RJ, Johnson DB. 2020. Sulfur-enhanced reductive bioprocessing of cobalt-bearing materials for base metals recovery. Hydrometallurgy, 195, 105396. doi: 10.1016/j.hydromet.2020.105396.
|
|
Shi ZY, Liu ZG, Xia CH. 2019. The present state of global nickel laterite resource development and the selection of smelting process. Jinchuan Keji, (2), 4–9 (in Chinese with English abstract) .
|
|
Shofi A, Rahmahwati A, Nurjaman F, Suharno B. 2019. Effect of reduction temperature and sodium-based additives on nickel upgrading process of laterites ores. IOP Conference Series: Materials Science and Engineering, 541(1), 012002. doi: 10.1088/1757-899x/541/1/012002.
|
|
Stanković S, Goldmann S, Kraemer D, Ufer K, Schippers A. 2024. Bioleaching of a lateritic ore (Piauí, Brazil) in percolators. Hydrometallurgy, 224, 106262. doi: 10.1016/j.hydromet.2024.106262.
|
|
Stanković S, Martin M, Goldmann S, Gäbler HE, Ufer K, Haubrich F, Moutinho VF, Giese EC, Neumann R, Stropper JL, Stummeyer J, Kaufhold S, Dohrmann R, Oxley A, Marbler H, Schippers A. 2022. Effect of mineralogy on Co and Ni extraction from Brazilian limonitic laterites via bioleaching and chemical leaching. Minerals Engineering, 184, 107604. doi: 10.1016/j.mineng.2022.107604.
|
|
Stanković S, Stopić S, Sokić M, Marković B, Friedrich B. 2020. Review of the past, present, and future of the hydrometallurgical production of nickel and cobalt from lateritic ores. Metallurgical and Materials Engineering, 26(2), 199–208. doi: 10.30544/513.
|
|
Suharno B, Nurjaman F, Ramadini C, Shofi A. 2021. Additives in selective reduction of lateritic nickel ores: Sodium sulfate, sodium carbonate, and sodium chloride. Mining, Metallurgy & Exploration, 38(5), 2145–2159. doi: 10.1007/s42461-021-00456-1.
|
|
Taylor MP, Gillings MM, Fry KL, Barlow CF, Gunkel-Grillion P, Gueyte R, Camoin M. 2023. Tracing nickel smelter emissions using European honey bees. Environmental Pollution, 335, 122257. doi: 10.1016/j.envpol.2023.122257.
|
|
Tian QH, Dong B, Guo XY, Xu ZP, Wang QA. 2021. A low-cost, low-acid consumption leaching method for laterite nickel ore. Chinese patent, CN202111641571. X (in Chinese).
|
|
Tian QH, Li ZC, Wang QM, Wang SS, Guo XY. 2023. Present situation of laterite nickel ore resources and research progress of smelting technology. The Chinese Journal of Nonferrous Metals, 33(9), 2975–2997 (in Chinese with English abstract). doi: 10.11817/j.ysxb.1004.0609.2022-43438.
|
|
Tong XW and Li YG. 2011. Engineering application of oxygen side-blown nickel smelting process in China. Hunan Nonferrous Metals, 27(3): 26−27.
|
|
Tsymbulov LB, Knyazev MV, Sh Tsemekhman L. 2011. Oxide nickel ores smelting of ferronickel in two-zone Vaniukov Furnace. Canadian Metallurgical Quarterly, 50(2), 135–144. doi: 10.1179/000844311x12949291727772.
|
|
Wang CY, Cao ZH, Ma BZ, Chen YQ. 2019. Nitric acid pressure leaching of laterite ores. The Chinese Journal of Process Engineering, 19(S1), 51–57 (in Chinese).
|
|
Wang QM, Chen YL, Guo XY, Wang SS, Tian QH. 2020. A method for extracting metallic nickel from laterite nickel ore. Chinese patent, CN202010533112.9 (in Chinese).
|
|
Wang S, Jiang Y, Zheng FQ, Chen F, Yang LZ, Guo YF. 2021. Development of pyrometallurgical technology of laterite nickel ore. China Metallurgy, 31(10), 1–7 (in Chinese with English abstract). doi: 10.13228/j.boyuan.issn1006-9356.20210291.
|
|
Wang YY, Wang S, Cui WY, Li YL, Guo Q, Kang JY. 2023. A coupled process of chloridizing leaching and selective hydrolysis for extracting nickel and cobalt from limonite-type and serpentine-type nickel laterite ores. Mining and Metallurgical Engineering, 43(3), 115–118,123 (in Chinese with English abstract). doi: 10.3969/j.issn.0253-6099.2023.03.026.
|
|
Wang HY, Li Y, Jiao SQ, Zhang GH. 2023. Preparation of Ni–Fe–S matte and Fe–Cr–Si alloy by the co-treatments of stainless steel pickling sludge and electroplating sludge. Metallurgical and Materials Transactions B, 54(6), 3229–3239. doi: 10.1007/s11663-023-02903-4.
|
|
Wang L, Lü X, Liu M, You Z, Lü X, Bai C. 2018. Preparation of ferronickel from nickel laterite via coal-based reduction followed by magnetic separation. International Journal of Minerals, Metallurgy, and Materials, 25, 744–751.
|
|
Wang XP, Sun TC, Kou J, Li ZC, Tian Y. 2018. Feasibility of co-reduction roasting of a saprolitic laterite ore and waste red mud. International Journal of Minerals, Metallurgy, and Materials, 25(6), 591–597. doi: 10.1007/s12613-018-1606-7.
|
|
Wang X, Zhu DQ, Guo ZQ, Pan J, Lv T, Yang CC, Li SW. 2023. Efficient utilization of limonite nickel laterite to prepare ferronickel by the selective reduction smelting process. Sustainability, 15(9), 7147. doi: 10.3390/su15097147.
|
|
Wei WJ, Samuelsson PB, Tilliander A, Gyllenram R, Jönsson PG. 2020. Energy consumption and greenhouse gas emissions of nickel products. Energies, 13(21), 5664. doi: 10.3390/en13215664.
|
|
Widyartha B, Setiyorini Y, Abdul F, Subakti TJ, Pintowantoro S. 2020. Effective beneficiation of low content nickel ferrous laterite using fluxing agent through Na2SO4 selective reduction. Materialwissenschaft and Werkstofftechnik, 51(6), 750–757. doi: 10.1002/mawe.202000007.
|
|
Wu BQ, Qi YH, Zhou HM, Hong LK, Zou ZS. 2020. Status and progress in pyrometallurgy processes of a laterite nickel ore. Multipurpose Utilization of Mineral Resources, (3), 78–83,93 (in Chinese). doi: 10.3969/j.issn.1000-6532.2020.03.012.
|
|
Wu BQ, Qi YH, Zhou HM, Wan XY, Zou ZS. 2019. Status and prospect analysis of hydrometallurgical processes of laterite nickel ore. China Metallurgy, 29(11), 1–5 (in Chinese with English abstract). doi: 10.13228/j.boyuan.issn1006-9356.20190198.
|
|
Xiao JH, Ding W, Peng Y, Chen T, Zou K, Wang Z. 2020. Extraction of nickel from garnierite laterite ore using roasting and magnetic separation with calcium chloride and iron concentrate. Minerals, 10(4), 352. doi: 10.3390/min10040352.
|
|
Xu CL, He YX, Wang XM, Liu DK, Liu BC, Qi SB. 2021. Development of nickel laterite ore leaching by nitric acid. World Nonferrous Metals, (12), 133–135 (in Chinese with English abstract). doi: 10.3969/j.issn.1002-5065.2021.12.062.
|
|
Yang XS, Guo YS, Chen BY, Cui YL & Guo X. (2013). The distribution and the exploration, development and utilization situation of the lateritic nickel ore resources in the world. Acta Geoscientica Sinica, 34(S1), 193−201 (in Chinese with English abstract) . doi: 10.3975/cagsb.2013.s1.30
|
|
Yu DW, Guo XY, G XD, Tian QH, Wang JJ. 2020. A method for separating nickel and iron from a nickel-iron alloy . Chinese patent, CN202011210440.1 (in Chinese).
|
|
Yu HJ, Xie YH, Li AX. 2022. Analysis of global patent information about metallurgical processing of nickel laterite. Mining and Metallurgical Engineering, 42(2), 96–101 (in Chinese with English abstract). doi: 10.3969/j.issn.0253-6099.2022.02.024.
|
|
Yuan JH. 2022. Current status and outlook of side blowing furnace. Nonferrous Metals (Extractive Metallurgy), (1), 31–35 (in Chinese with English abstract). doi: 10.3969/j.issn.1007-7545.2022.01.006.
|
|
Zappala L, McDonald R, Pownceby MI. 2023. Nickel laterite beneficiation and potential for upgrading using high temperature methods: A review. Mineral Processing and Extractive Metallurgy Review, 1–3. doi: 10.1080/08827508.2023.2265533.
|
|
Zevgolis EN, Daskalakis K. 2022. The nickel production methods from laterites and the Greek ferronickel production among them. Materials Proceedings, .
|
|
Zhang ZF, Chen XF, Li YC, Gao AH, Wang YG, He XZ, Wang QS. 2022. Multipurpose utilization trend of nickel mineral resources under the goal of carbon peaking and carbon neutrality. Multipurpose Utilization of Mineral Resources, (2), 31–39 (in Chinese with English abstract).
|
|
Zhang PY, Sun LQ, Wang HR, Cui JW, Hao JC. 2019. Surfactant-assistant atmospheric acid leaching of laterite ore for the improvement of leaching efficiency of nickel and cobalt. Journal of Cleaner Production, 228, 1–7. doi: 10.1016/j.jclepro.2019.04.305.
|
|
Zhao D, Ma BZ, Wang CY, Chen YQ. 2023. Research progress of limonitic laterite hydrometallurgy. Journal of Central South University (Science and Technology), 54(2), 401–414 (in Chinese). doi: 10.11817/j.issn.1672-7207.2023.02.001.
|
|
Zulhan Z, Shalat W. 2021. Evolution of ferronickel particles during the reduction of low-grade saprolitic laterite nickel ore by coal in the temperature range of 900–1250°C with the addition of CaO-CaF2-H3BO3. International Journal of Minerals, Metallurgy and Materials, 28(4), 612–620. doi: 10.1007/s12613-020-2025-0.
|
| [1] | Cong Chen, Yu-chao Gu, Di Zhang, Tao-tao Wu, Ai Li, Yun-sheng Ren, Qing-qing Shang, Jian Zhang, Xiong-fei Bian, Fei Su, Jia-lin Yang, Qiu-shi Sun, Xiao-hai Li, Wan-zhen Liu, Zhen-ming Sun, Sen Zhang, Yu-hui Feng. 2024: Geology and mineralization of the Hongqiling large magmatic nickel-copper-cobalt deposit (22×104 t) in Jilin Province, China: A review. China Geology, 7(4): 762-796. doi: 10.31035/cg2023106 |
| [2] | Fan Yang, Jing-wen Mao, Wei-dong Ren, Jia-run Tu, Gilby Jepson, Si-yuan Meng, Zhi-min Wang. 2024: In situ U-Pb dating and trace elements of magmatic rutile from Mujicun Cu-Mo deposit, North China Craton: Insights into porphyry mineralization. China Geology, 7(4): 730-746. doi: 10.31035/cg2023038 |
| [3] | Zhi-qiang Yang, Wen-wen Qi, Chong Xu, Xiao-yi Shao. 2024: Exploring deep learning for landslide mapping: A comprehensive review. China Geology, 7(2): 330-350. doi: 10.31035/cg2024032 |
| [4] | Bin Lin, Ju-xing Tang, Pan Tang, Wen-bao Zheng, Yang Song, Fa-qiao Li, Qiu-feng Leng, Zhi-chao Wang, Jing Qi, Miao Sun, Juan David Bello Rodríguez. 2023: Geology, geochronology, and exploration of the Jiama giant porphyry copper deposit (11 Mt), Tibet, China: A review. China Geology, 6(2): 338-357. doi: 10.31035/cg2023031 |
| [5] | Hua-yun Yuan, Qing Zhou, Yuan-bao Song, Wei Zhang, Hui-hua Zhang, Tong-zhu Li, Tao Yin, Chang-nan Wang, Gao-lin Tang. 2023: Mineralization of the Liwu large-scale stratiform copper deposits in Sichuan Province, China: Constraints from fluid inclusions. China Geology, 6(2): 252-268. doi: 10.31035/cg2023034 |
| [6] | Tian-ming Gao, Na Fan, Wu Chen, Tao Dai. 2023: Lithium extraction from hard rock lithium ores (spodumene, lepidolite, zinnwaldite, petalite): Technology, resources, environment and cost. China Geology, 6(1): 137-153. doi: 10.31035/cg2022088 |
| [7] | Wen-chang Li, Xiang-fei Zhang, Hai-jun Yu, Dong Tao, Xue-long Liu. 2022: Geology and mineralization of the Pulang supergiant porphyry copper deposit (5.11 Mt) in Shangri-la, Yunnan Province, China: A review. China Geology, 5(4): 662-695. doi: 10.31035/cg2022060 |
| [8] | Huan-huan Yang, Qin Wang, Yan-bo Li, Bin Lin, Yang Song, Yi-yun Wang, Wen He, Hong-wei Li, She Li, Jian-li Li, Chang-cheng Liu, Shi-bin Feng, Tang Xin, Xue-lian Fu, Xin-juan Liang, Qi Zhang, Bei-qi Wang, Yang Li. 2022: Geology and mineralization of the Tiegelongnan supergiant porphyry-epithermal Cu (Au, Ag) deposit (10 Mt) in western Tibet, China: A review. China Geology, 5(1): 136-159. doi: 10.31035/cg2022001 |
| [9] | Bang-jun Liu, Guang-chen Chu, Cun-liang Zhao, Yu-zhuang Sun. 2022: Leaching behavior of Li and Ga from granitic rocks and sorption on kaolinite: Implications for their enrichment in the Jungar Coalfield, Ordos Basin. China Geology, 5(1): 34-45. doi: 10.31035/cg2021024 |
| [10] | Bai-dong Sun, Jun-ping Liu, Xiao-hu Wang, Yan Dao, Gui-xiang Xu, Xiao-zhuang Cui, Xue-qing Guan, Wei Wang, Dong-hu Song. 2019: Geochemical characteristics and genetic type of a lithium ore (mineralized) body in the central Yunnan Province, China. China Geology, 2(3): 287-300. doi: 10.31035/cg2018118 |
| Country | Reserves/Mt | Global share/% | Resources (including reserves) /Mt) | Global share/% |
| Indonesia | 21 | 21 | 71 | 19 |
| Australia | 21 | 21 | 40 | 11 |
| Brazil | 16 | 16 | 19 | 5 |
| Russia | 7.5 | 7.5 | 36 | 10 |
| New Caledonia | 7.1 | 7.1 | 31 | 8 |
| The Philippines | 4.8 | 4.8 | 31 | 8 |
| Canada | 2.2 | 2.2 | 39 | 11 |
| China* | 2.1 | 2.1 | 9 | 2 |
| Others | 20 | 20 | 89 | 24 |
| Total | >100 | 100 | 365 | 100 |
| Data source: Reserve data come from USGS Mineral Commodity Summaries 2023, resource data come from S&P Global Market Intellgence Mineral Database (data as of May 2023). *According to the China Mineral Resources Report (2022), China's nickel reserves are 4.22×106 t. | ||||
| Ore type | Mass fraction/% | Characteristics | Extraction process | |||||
| Ni | TFe | MgO | SiO2 | Co | Cr2O3 | |||
| Limonite | 0.8‒1.4 | 36‒50 | 0.5‒5.0 | 0‒10 | 0.1‒0.2 | 2‒5 | Low Ni & Mg, high Fe | Hydrometallurgy |
| Nontronite | 1.2‒1.8 | 25‒40 | 5‒15 | 10‒30 | 0.02‒0.1 | 1‒2 | Pyrometallurgy/ Hydrometallurgy |
|
| Saprolite | 1.4‒3.0 | 10‒25 | 15‒35 | 30‒50 | 0.02‒0.1 | 1‒2 | High Ni, Mg &Si, low Fe | Pyrometallurgy |
| Type | Process | Examples | Feed type | Lixiviant | T(°C)/ P(atm) |
Time | Advantages | Disadvantages | Product | Extraction/% | Recovery/% | Embodied energy (GJ/t Ni) |
GHG emissions (t CO 2 eq/t Ni) |
References |
| Hydrometallurgical process | High Pressure Acid Leaching (HPAL) | Ramu, Goro, Ambatovy | Limonite, w(Mg)<5%w(Ni)> 1.3%) |
H2SO4 HNO3 H3PO4 |
250‒270; 30‒50 atm | 90 min | Low energy consumption, low GHG emissions, high recovery | High equipment requirements, harsh operation, and high capital expenditure | Intermediate | 90‒95 | Ni: 95; Co: 90 | 272 | 19 | IEA, 2021; Pandey N et al., 2023; Stanković S et al., 2020 |
| Atmospheric leaching (AL) | Eaglebridge’s Kristiansund refinery in Norway | Limonite | H2SO4 HCl HNO3 |
85‒110; 1atm | 12 h | Low energy consumption, easy to operate and control | Low extraction, long leaching time and high acid consumption | Intermediate | 85‒95 | Ni: 98.2; Co: 98.6 | 167 | 14.6 | Pandey N et al. 2023; Stanković S et al., 2020; Tian QH et al., 2023 | |
| Heap Leaching (HL) | Piaui (Brazil) NiWest (Australia) Acoje (Philippines) Pearl (Indonesia) | Limonite-Saprolite | H2SO4 | Ambiental | 120‒ 150 d |
Low capital and operating costs, low energy consumption, easy operating | low recovery, higher acid consumption | Intermediate | 70‒80 | Ni: 86 | 211 | 17.6 | Pandey N et al., 2023; Stanković S et al., 2020 | |
| Direct Nickel (Dni) | Dni built a pilot test line in Perth, Australia; Altilium of UK plans to build a smelter in Indonesia | Saprolite | HNO3 | 105°C; 1atm | 2‒4 h | Simple plant maintenance, flexibility in feed, recycled nitric acid |
Strict acidity control, removal of dissolved Al during purification |
Intermediate | >90 | Ni: 100 | 380‒400 | 34‒36 | Pandey N et al., 2023; Stanković S et al., 2020; Khoo JZ et al., 2017; Zhao D et al., 2023 | |
| Pyrometallurgical Process | Rotary kiln - electric furnace (RKEF) | Sorowako/Pomalaa (Indonesia), Tagaung Taung (Myanmar), Dingxin (Tingshan) Cerro Matoso (Colombia) | Saprolite w(Ni) about 2%, w(Fe)< 20%) |
/ | 850–1000°C in Rotary Kiln 1500‒ 1600°C in Electric Furnace |
/ | High nickel recovery, short process and high efficiency | High energy consumption, large amount of slag, poor ore adaptability, high operating cost | ferronickel | / | Ni>90; Fe>90 | 592, 35000‒ 40000 kWh/t of nickel |
45 | Wei WJ et al., 2020; Nickel Institut, 2023; Tian QH et al., 2023; Shi ZY et al., 2019; Wang S et al., 2021 |
| Oxygen-enriched side-blown (OESB) | Morowali (Indonesia) | Limonite & Saprolite, | / | 1500°C; 1atm | / | Low cost, short construction period, flexible ferronickel grade, flexibility in feed, environmentally friendly |
The technology is difficult and the production efficiency remains to be seen | Nickel matte | / | Ni: 94.12; Fe: 52 | 10000 kWh/t of nickel | 33.75 | Chen XG et al., 2018 | |
| Sulfide smelting | Donniambo (New Caledonia) Soroako (Indonesia) |
Saprolite, strict requirements on magnesium content | / | 1500‒ 1600 |
/ | Mature technology, low equipment cost, easy operation, flexible product form | High energy consumption, high GHG, low recovery, poor feed adaptability | Nickel matte | / | Low nickel matte Ni: 8‒15 High nickel sulfur Ni: 70 | 59 | IEA, 2021; Wang S et al., 2021; Wu BQ et al., 2020; Li JH et al., 2015 | ||
| Pyro-hydrometallurgical combined process | Reduction roasting-ammonia leaching (Caron) | Yabulu (Austrila), Surigao&Berong (Philippines), Sukhinda (India), Yuanshishan (China) | Limonite & Saprolite, w(Mg)>10% | NH3-(NH4)2CO3 | 600‒900 °C; 1atm |
/ | Recyclable leaching agent, low cost | Low recovery, high energy consumption, poor operating environment | Nickel Oxide, Nickel Sulfate | Ni: 75‒80; Co<50 | Ni:91.2; Co:67.2 | 565 | 44.8 | Tian QH et al., 2023; Zhao D et al., 2023; Pandey N et al., 2023; Stanković S et al., 2020 |
| Selective reduction-hydrometallurgical magnetic separation (Krupp-Renn) | Oyama (Closed); Beihai Chengde Nickel Company | Saprolite w(Ni)>1.8% |
/ | 1400°C | / | Low energy consumption and low operating cost | Rotary kiln ringing, difficult operation, limited production | Nickel pig iron | / | Ni: 20‒25; Fe: 75-80 | Coal consumption per ton of ore: 170 tons 5000 kWh/ton of nickel | Tian QH et al., 2023; Wu BQ et al., 2020 | ||
| Other processes | Ferredox reductive bioleaching | Piaui (Brazil) (concept) | Limonite | H2SO4+acidophilic bacteria | 30°C; 1atm | 7d | High cobalt recovery, lower capital and operational expenses, lower CO2 emissions |
Lower process efficiency, lower nickel extraction |
/ | Ni: 66; Co: 95; Fe: 10; Mg: 50; Mn: 81 | Stanković S et al. 2020, 2024; Li JH et al., 2015 | |||
| Chloride Metallurgy | Limonite & Saprolite, | HCl | 100°C; 1atm | 30 min/ 90 min |
High nickel and cobalt extraction, relatively mild leaching conditions, acid and alkali renewable | Higher iron extraction, high acid consumption, highly corrosive hydrochloric acid requires a tougher equipment. | / | Limonite Ni: 99.6; Co: 100; Fe: 96.9; Saprolite Ni: 84.9; Co: 100 | Zhao D et al., 2023; Wang YY et al., 2023; Li JH et al., 2015; Stanković S et al., 2020 |
| Company Name | Plants or projects/country | Process and products | Nickel-cobalt production scale/kt |
Investment amount (B$) and start-up time |
| Sherritt | Cuba/Moa | HPAL/MSP | 32; 2 | -; 1959 |
| Glencore | Murrin Murrin/Australia | HPAL/MSP | 36.6; 3 | 1; 1998 |
| Sumitomo Metal | Rio Tuba (Coral)/Philippines | HPAL/MSP | 22; 1.5 | 0.487; 2005 |
| First Quantum | Ravensthorpe/Australia | HPAL/MHP | 25; 14 | 2.2; 2008 |
| Prony Resources | Goro/New Caledonia | Nickel Oxide & Cobalt Carbonate | 60; 5 | 5.8; 2009 |
| MCC | Ramu/Papua New Guinea | HPAL/MHP | 32; 3 | 2.04; 2012 |
| Sumitomo Metal/Sherritt | Ambatovy/Madagascar | Nickel & Cobalt Briquettes | 60; 5 | 5.5; 2012 |
| Sumitomo Metal | Taganito/Philippines | HPAL/MSP | 36; 2.6 | 1.7; 2013 |
| Liqin/Harita | Obi Island/Indonesia | HPAL/MHP | 37; 4.5 | 0.7; 2021 |
| Huayou / Tsingshan | Morowali/Indonesia | HPAL/MHP | 60; 7.8 | 1.28; 2022 |
| Tsingshan/Gelinmei/Bangpu | Morowali/Indonesia | HPAL/MHP | 50; 4 | 0.998; 2022 |
| Huayou/Tsingshan/Yiweilinng | Weda Bay/Indonesia | HPAL/MHP+MSP | 120; 15 | 2.08; 2023 |
| Eramet/BASF | Weda Bay/Indonesia | HPAL/MHP | 42; 5 | -; 2025 |
| Vale/Sumitomo Metal | Pomalaa/Indonesia | HPAL/MSP | 40; - | -; 2026 |
| Acid solution | Advantages | Disadvantages |
| Sulfuric acid | Direct heap leaching, operate easily | Large acid consumption acid can not be recycled, low leaching efficiency |
| Nitric acid | High Ni leaching rate, nitric acid can be recycled | Prone to produce toxic gas NOx |
| Hydrochloric acid | The separation of Ni, Co is easy, hydrochloric acid can be recycled | High corrosiveness, high requirements of equipment |
| Organic acid & microbial derived acid | Low leaching temperature, operate easily and environmental friendliness | Long operating time, low efficiency |
| Project | Country | Owner | Estimated cost USM$ | Planned Ni production capacity(ktpy) | Capital intensity US$/lb Ni |
| Piaui | Brazil | Brazilian Nickel | 450 | 22 | 9.28 |
| NiWest | Australia | GME | 400 | 14 | 12.75 |
| Cerro Matoso | Colombia | BHPB | 750 | 20 | 17.01 |
| Caldag | Turkey | ENK | 450 | 20 | 10.30 |
| – | Guatemala | BHPB | 2550 | 79.5 | 15.44 |
| Pearl | Indonesia | BHPB | 800 | 32 | 11.11 |
| Gag Island | Indonesia | BHPB | 800 | 27.3 | 13.47 |
| Cleopatra | USA | RFN | 475 | 21.5 | 10.02 |
| Acoje | Philippines | ENK | 498 | 24.5 | 9.22 |
| Property | Country | Owner Name | Start Up | Capacity/kt Ni | Production Forms | Processing |
| Codemin | Brazil | Anglo American plc | 1982 | 10 | Fe-Ni | Smelting |
| Barro Alto | Brazil | Anglo American plc | 2011 | 41 | Fe-Ni | RKEF |
| Onca Puma | Brazil | Vale SA | 2011 | 27 | Fe-Ni | RKEF |
| Cerro Matoso | Colombia | South32 Limited | 1982 | 50 | Fe-Ni | Smelting |
| Falcondo | Dominican | Global Special Opportunities Ltd. | 1971 | 29 | Fe-Ni | Smelting |
| Pomalaa | Indonesia | PT Aneka Tambang Tbk | 1975 | 27 | Fe-Ni | Smelting |
| Weda Bay/Youshan | Indonesia | Chengtun Mining Group Co., Ltd. | 2020 | 34 | Fe-Ni | RKEF, Blast Furnace |
| SLN/Doniambo | New Caledonia | ERAMET SA | 1879 | 55 | Matte; Fe-Ni | Smelting |
| Koniambo | New Caledonia | Societe Miniere du Sud Pacifique SA | 2013 | 55 | Alloy | Smelting |
| Larco/Larymna plant | Greece | Larco SA | 1994 | 25 | Fe-Ni | RKEF |
| Tagaung Taung Smelter | Myanmar | China Nonferrous Metal Mining (Group) Co., Ltd. | 2011 | 22 | Fe-Ni | RKEF |
| Hachinohe Smelter | Japan | Pacific Metals Co., Ltd. | 1966 | 40.8 | Fe-Ni | RKEF |
| Hyuga Smelter | Japan | Sumitomo Metal Mining Co., Ltd. | 1956 | 22 | Fe-Ni | Smelting |
| Loma de Niquel Smelter | Venezuela | Federal Corporacion De Minas | 2000 | 17 | Fe-Ni | Smelting |
| Pobuzhskiy Plant | Ukraine | Solway Investment Group Limited | 2003 | 22 | Fe-Ni | Smelting |
| Sorowako | Indonesia | PT Vale Indonesia Tbk | 1977 | 80 | Matte | Smelting |
| Morowali/ZhongTsing Smelter | Indonesia | CNGR Advanced Material Co.,Ltd. | 2023 | 30 | Matte | Oxygen-enriched side blowing |
| Oheyama Smelter | Japan | Nippon Yakin Kogyo Co., Ltd. | 1939 | 10 | Fe-Ni | Krupp-Renn |
| Piaui | Brazil | Brazilian Nickel Plc | 2016 | 1 | Hydroxide | Acid Leach |
| Punta Gorda | Cuba | Cubanique | 1986 | 30 | Ni Oxide | Caron |
| Nicaro Smelter | Cuba | General Nickel Co SA | 1981 | 15 | NiO | Caron |
| Yabulu Refinery | Australia | Queensland Nickel Pty Ltd. | 1974 | 32 | Hydroxide | Caron |
| Ore type | Nation/Area | Ni% | Tfe% | Reducing Agents | Additives | Reduction temperature | Reduction time | Nickel grade/recovery | Iron grade/recovery | Source |
| Limonite | Sulawesi | 1.59 | 42.01 | Sub-bituminous coal | CaCO311%+Na2SO4 10% | 700; 1400 | 2 h; 6 h | 18.75; 99.34 | 52.95; 18.78 | Abdul F et al., 2023 |
| Limonite | Indonesia | 1.25 | 55.37 | Sub-bituminous coal | Na2SO4 +CaCO3 | 1400 | 6 h | Grade 21.68 (limestone); Recovery 93.73 (dolomite) | 63.7 (Direct reduction using Na2SO4); - | Widyartha B et al., 2020 |
| Limonite | Sulawesi | 1.59 | 42.01 | Sub-bituminous coal + limestone | Na2S2O3 10% | 700; 1400 | 2 h; 6 h | 14.31; 93.22 | 64.07; 19.02 | Pintowantoro S et al., 2021b |
| saprolite | Indonesia | 1.41 | 24.14 | H2 | Na2S2O3 20% | 1100 | 90 min | 9.97; 99.24 | 44; 35 | Liu S et al.; 2021 |
| Limonite | 1.38 | 56 | Coal 6% | S 4% | 600; 1000 | 1 h; 1 h | 4; 93.2 | 96; - | Elliott R et al., 2015 | |
| Limonite | Sulawesi | 1.59 | 42.01 | Sub-bituminous coal + limestone | S 10% | 700; 1400 | 2 h; 6 h | 13.62; 97.91 | 53.39; 22.18 | Pintowantoro S et al., 2021b |
| Limonite | Philippines | 1.49 | 34.69 | Coal 2% | S 10% | 1200 | 50 min | 7.21; 73.07 | 68.01; 29.43 | Jiang M et al.; 2013 |
| Limonite | Indonesia | 1.16 | 46.55 | Straw charcoal 15% | Red mud 25% | 1250 | 80 min | 1.81; 97.21 | 81.4; 98.87 | Guo X et al.; 2021 |
| Limonite | 1.62 | 14.7 | Anthracite 10% | Red mud 35% | 1200–1300 | 60 min | 4.9; 95.25 | 71; 93.77 | Wang XP et al.; 2018 | |
| Limonite | Sulawesi | 1.38 | 41.06 | Bituminous coal | Na2SO4 | 1250 | 1 h | 7.3; 92 | -; 34 | Nurjaman F et al.; 2021 |
| saprolite | Sulawesi | 1.81 | 18.48 | Bituminous coal | Na2SO4 | 1250 | 1 h | 12.3; 35 | -; 19 | Nurjaman F et al.; 2021 |
| Limonite | Philippines | 1.49 | 34.69 | Coal 2% | Na2SO4 10 % | 1200 | 50 min | 9.87; 90.90 | 72.05; 29.55 | Jiang M et al., 2013 |
| Limonite | Sulawesi | 1.4 | 50.5 | Palm Shell Charcoal 5% | Na2SO4 10 % | 1150 | 1 h | 4.6; 73.2 | 81.9; 35 | Shofi A et al., 2019 |
| Limonite | Indonesia | 1.4 | 50.5 | Anthracite 5% | Na2SO4 15 % | 1150 | 60 min | 15.06; 65 | 60.58; 7.5 | Suharno B. et al., 2021 |
| saprolite | Indonesia | 1.91 | 22.1 | Lignite | Na2SO4 20 % | 1100 | 60 min | 9.48; 83.01 | 79.3; 56.36 | Li G et al., 2012 |
| Limonite | Indonesia | 1.4 | 50.5 | Anthracite 5% | NaCl 15% | 1050 | 60 min | 2; 85.79 | 61.53; 74.67 | Suharno B et al., 2021 |
| Limonite | 1.13 | 35.79 | Coal 10% | NaCl 15% | 1250 | 80 min | 8.15; 97.76 | 64.28; - | Qu GR.et al., 2019 | |
| Limonite | Indonesia | 1.4 | 50.5 | Anthracite 5% | Na2CO3 15% | 1150 | 60 min | 2.18; 67.01 | 55.84; 71.4 | Suharno B et al., 2021 |
| Limonite | Sulawesi | 1.31 | 8.3 | Coal 10% | 10% CaO + 10% CaF2+H3BO3 10% | 1250 | 2 h | 8.59; - | 85.65; - | Zulhan Z and Shalat W, 2021 |
| Limonite | Sulawesi | 1.4 | 16.18 | Coal | CaF2 8% | 1250 | 50 min | 7.1; 84.14 | 68.5; 70.24 | Hang G, 2021 |
| Limonite | Halmahera | 1.29 | 27.02 | Coal 10% | CaSO4 10% | 1100 | 60 min | 4.34; 50.38 | 57.48; 37.58 | Mayangsari W et al., 2018 |
| Limonite | Philippines | 1.49 | 34.69 | Coal 2% | S+Na2O 10 % | 1200 | 50 min | 9.29; 87.29 | 76.22; 30.76 | Jiang M et al., 2013 |
| Limonite | 1.59 | 42.01 | Coal | CaSO4 12% | 1400 | 6 h | 4.13; 91.81 | 27.33; - | Pintowantoro S et al., 2021a | |
| Limonite | Philippines | 0.98 | 48.09 | Limestone +dolomite +slaked lime | 1525 | 45 min | 12.55; 85.65 | 84.61; 10.87 | Wang X et al., 2023 |
| 1 kg Class 1 Ni | 1 kg Ni in FeNi (27% Ni in FeNi) | 1 kg NiSO4 | |
| Global Warming Potential (GWP) [kg CO 2 eq.] | 13 | 45 | 4 |
| Acidification Potential [kg SO 2 eq.] | 1.4 | 0.17 | 0.26 |
| Primary Energy Demand (PED) [MJ] | 236 | 592 | 68 |
| Eutrophication Potential [kg Phosphate eq.] | 5.2E-03 | 0.016 | 1.5E-03 |
| Photochemical Ozone Creation Potential [kg Ethene eq.] | 0.055 | 0.010 | 0.010 |
| Blue water consumption [kg] | 106 | 924 | 49 |
| Impact category (unite) | Significant process (% of impact category) | Significant contributor to the process (% of process) | Significant flow (% of impact category) |
| PED (MJ) | Primary extraction (70%) | Electricity (35%) | Crude oil (43%) natural gas (17%) |
| GWP (kg CO2 -equivalents) | Primary extraction (65%) | Electricity (48%) | Carbon dioxide (–100%) |
| Ore type | Nickel sulfide ore | Nickel laterite | Nickel laterite | Nickel laterite | |
| Product type | Nickel metal 100%Ni |
NiO (76% Ni) | FeNi (35% Ni) | NPI (10% Ni) | |
| The most energy-consuming sub- processes | Refining | Smelting | Calcination | Smelting | |
| Energy consumption contribution of the sub- processes to the process (%) | 44 | 45 | 44 | 67 | |
| Carbon emissions contribution of the sub- processes to the process (%) | 38 | 45 | 55 | 73 | |
| Carbon emission sources and energy of the sub- processes | Electricity | Fossil fuels and reducing agents | Fossil fuels and reducing agents | Fossil fuels and reducing agents | |
| Mixed energy | 60% coal | 49% oil | 70%hydro | 49% coal | |
| Energy consumption per ton of nickel product (GJ) | 174 | 370 | 110 | 60 | |
| Energy consumption per ton of nickel metal (GJ) | 174 | 485 | 309 | 598 | |
| Carbon emissions per ton of nickel product (tCO2 -eq) | 14 | 30 | 6 | 7 | |
| Carbon emissions per ton of nickel metal (tCO2 -eq) | 14 | 40 | 18 | 69 | |
| 100% hydro-power electricity | |||||
| Energy consumption per ton of nickel product (GJ) | 127 | 270 | 102 | 59 | |
| Carbon emissions per ton of nickel product (tCO2 -eq) | 7 | 17 | 4.9 | 7 | |
| Deduction for recycling, etc. | |||||
| Energy consumption per ton of nickel product (GJ) | 116 | 235 | 61 | 39 | |
| Energy consumption per ton of nickel metal (GJ) | 116 | 309 | 172 | 385 | |
| Carbon emissions per ton of nickel product (tCO 2 -eq) | 6 | 18 | 4 | 6 | |
| Carbon emissions per ton of nickel metal (tCO 2 -eq) | 6 | 24 | 10 | 57 | |
| Content of heavy metal(loid)s (mg/kg) in slag and leaching residue from Ni laterite | |||||||
| element | Slag from Ni laterites | Leaching residue from Ni laterite | |||||
| Min | Max | Average | Min | Max | Average | ||
| As | 3.3 | 26.2 | 11.8 | 25 | 425 | 300 | |
| Co | 40 | 220 | 115 | 10 | 890 | 218 | |
| Cr | 6340 | 8000 | 6848 | 4600 | 17400 | 9150 | |
| Cu | 6.23 | 1157 | 399 | 36 | 320 | 127 | |
| Mn | 2650 | 3500 | 3007 | 100 | 8150 | 2558 | |
| Ni | 740 | 3200 | 1303 | 330 | 3400 | 1763 | |
| Zn | 85 | 1162 | 357 | 130 | 480 | 300 | |
| Cd | 0.25 | 4.58 | 2.4 | ||||
| Pb | 39 | 300 | 195 | ||||
| Sb | 26.2 | 79.2 | 45.8 | ||||
| V | 65.9 | 84.8 | 75.2 | ||||
| Processing Method | Production Forms | Head Grade Ni% | CapEx(k$/ t)Ni Capacity | OpEx ($/t) | Production Capacity(kt)Ni | Recovery Rate Ni% |
| HPAL | MHP/MSP | 1–1.8 | 56 | 15198 | 28–60 | 83–90 |
| RKEF | FeNi | 1.31–2.2 | 43 | 16278 | 10–55 | 77–90 |
| Smelting | Matte | 1.69 | 1 | 14583 | 80 | 88 |
| AL | Hydroxide | 1.3 | – | 12565 | 1 | 85 |
| Caron | Concentrate | 1.2 | 19 | 17611 | 30 | 70 |
| HL | Hydroxide | Saprolite-limonite | 20–33 | 4889–6667 | 10–60 | 86 |
| OESB | Matte | Saprolite-limonite | 22 | 7414–8081 | 30 | 93 |
| Note: HL data is from Oxley A et al., (2016), OESB production cost data is from Chen XG et al., 2018, and the rest data are from S&P laterite nickel ore economic model | ||||||
| Economic and environmental indicators | Ferronickel sulfidation blowing | Oxygen-enriched side blowing | High pressure acid leaching |
| Ni Capital intensity | 12 | 8.1 | 12–20 |
| Cash cost + maintenance expenditure of nickel sulfate (k$/t) | 13–14 | 11–12 | 9.7–10 |
| Construction period (year) | 2 | 1–2 | 2–3 |
| Raw material requirements | Humus soil type | Various types | Limonite type |
| Tailings (t slag/t Ni) | 6–16 | – | 150–200 |
| Energy consumption | 592 | – | 272 |
| Carbon emissions | 45 | – | 19 |